A first order reaction has rate constants of 4.6 x 10-2 s-1 and 8.1 x 10-2 s-1 at 0ºC and 20ºC, respectively. What is the value for the activation energy?
A.
0.566 J/mol
B.
2.5 x 10-4 J/mol
C.
2260 J/mol
D.
18,800 J/mol
E.
1.76 J/mol
Answers
Answer:
D. 18,800 J/mol
Explanation:
We need to use the Arrhenius equation to solve for this problem:
\(k=Ae^{\frac{-E_a}{RT}\), where k is the rate constant, A is the frequency factor, \(E_a\) is the activation energy, R is the gas constant, and T is the temperature in Kelvins.
We want to find the value of \(E_a\), so let's plug some of the information we have into the equation. The gas constant we can use here is 8.31 J/mol-K.
At 0°C, which is 0 + 273 = 273 Kelvins, the rate constant k is \(4.6*10^{-2}\). So:
\(k=Ae^{\frac{-E_a}{RT}\)
\(4.6*10^{-2}=Ae^{\frac{-E_a}{8.31*273}\)
At 20°C, which is 20 + 273 = 293 Kelvins, the rate constant k is \(8.1*10^{-2}\). So:
\(k=Ae^{\frac{-E_a}{RT}\)
\(8.1*10^{-2}=Ae^{\frac{-E_a}{8.31*293}\)
We now have two equations and two variables to solve for. We just want to find Ea, so let's write the first equation for A in terms of Ea:
\(4.6*10^{-2}=Ae^{\frac{-E_a}{8.31*273}\)
\(A=\frac{4.6*10^{-2}}{e^{\frac{-E_a}{8.31*273}} }\)
Plug this in for A in the second equation:
\(8.1*10^{-2}=Ae^{\frac{-E_a}{8.31*293}\)
\(8.1*10^{-2}=\frac{4.6*10^{-2}}{e^{\frac{-E_a}{8.31*273}} }e^{\frac{-E_a}{8.31*293}\)
After some troublesome manipulation, the answer should come down to be approximately:
Ea = 18,800 J/mol
The answer is thus D.
Related Questions
the gas in a 275.0 ml piston experiences a change in pressure from 1.00 atm to 3.10 atm. what is the new volume (in ml) assuming the moles of gas and temperature are held constant?
Answers
The new volume of the gas is 832.5 ml assuming the moles of gas and temperature are held constant
Since the temperature and number of moles of gas are held constant, the pressure-volume relationship of the gas follows Boyle's Law, which states that the pressure and volume of a gas are inversely proportional at constant temperature.
the gas in a 275.0 ml piston experiences a change in pressure from 1.00 atm to 3.10 atm.
P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
V2 = V1 * P1 / P2
= 275.0 ml * 1.00 atm / 3.10 atm
= 832.5 ml
hence, The new volume of the gas is 832.5 ml.
Learn more about volume here:
https://brainly.com/question/13338592
#SPJ4
How many grams of hydrogen must react to produce 31. 5 grams of ammonia?.
Answers
To determine how many grams of hydrogen must react to produce 31.5 grams of ammonia, we must use stoichiometry. Stoichiometry is the study of the quantitative relationships between reactants and products in a balanced chemical equation.
Ammonia is formed when hydrogen gas reacts with nitrogen gas according to the following balanced equation:N2(g) + 3H2(g) → 2NH3(g)From the balanced equation, we can deduce that three moles of hydrogen gas reacts with one mole of nitrogen gas to produce two moles of ammonia gas.
The molar mass of nitrogen gas (N2) is 28 g/molThe molar mass of hydrogen gas (H2) is 2 g/molThe molar mass of ammonia gas (NH3) is 17 g/molLet us first calculate the number of moles of ammonia formed when 31.5 grams of ammonia reacts.
To know more about grams visit:
https://brainly.com/question/30426054
#SPJ11
5. list the following alkyl halides in order of increasing sn1 reaction rate. what will be the reaction rate order for sn1 reaction rate? provide your reasoning.
Answers
The reaction rate order for sn1 reaction rate will be CH3F < CH3Cl < CH3I. The reason for this is that the halide with the weaker nucleophile will have a higher sn1 reaction rate.
The Effect of Nucleophile Strength on Sn1 Reaction RateThe nucleophile is a key factor in determining the rate of an Sn1 reaction. In general, the stronger the nucleophile, the faster the reaction will be. This is because the stronger nucleophile will be able to break the bond between the alkyl halide and the nucleophilic carbon more easily. For this reason, the order of increasing Sn1 reaction rate will be CH3F < CH3Cl < CH3I.
Complete Question:
List the following alkyl halides in order of increasing sn1 reaction rate. what will be the reaction rate order for sn1 reaction rate? provide your reasoning.
CH3FCH3ClCH3ILearn more about chemistry reactions:
https://brainly.com/question/16416932
#SPJ4
Mrs. Keep burns a walnut under a beaker of water. The beaker contains 100 g of water which warms from 25oC to 30oC. Assuming that all the heat from the burning walnut goes into the water and none of the heat is lost to the air or the beaker, how many calories are in the walnut?
a 2100 calories
b 10,500 calories
c not enough information is given
d 500 calories
Answers
The amount of heat gained by the water is 500 calories. Thus, option D is correct.
Given:
Mass of water (m) = 100 g
Change in temperature (ΔT) = 30°C - 25°C = 5°C
The specific heat capacity of water (c) is approximately 1 calorie/gram°C.
Now, the amount of heat gained by the water,
Q = mcΔT
Where:
Q is the heat gained or lost by the substance
m is the mass of the substance
c is the specific heat capacity of the substance
ΔT is the change in temperature
Plugging in the values into the formula:
Q = 100 × 1 × 5
Q = 500 calories
Therefore, the amount of heat gained by the water is 500 calories.
Learn more about heat, here:
https://brainly.com/question/31608647
#SPJ1
A 0.035 M solution of ammonia has a pH of 11.33. Calculate Kb for ammonia.
Answers
With the formula NH3, ammonia is a nitrogen and hydrogen inorganic chemical.
Thus, Ammonia, the simplest pnictogen hydride and a stable binary hydride, is a colourless gas with a strong, pungent odour. It contributes considerably to the nutritional demands of terrestrial creatures by serving as a precursor to 45% of the world's food and fertilizers.
Biologically, it is a common nitrogenous waste, especially among aquatic animals.
The production of fertilizers in a variety of shapes and compositions, including urea and diammonium phosphate, uses about 70% of ammonia.
Thus, With the formula NH3, ammonia is a nitrogen and hydrogen inorganic chemical.
Learn more about Ammonia, refer to the link:
https://brainly.com/question/31525313
#SPJ4
What Kind of model is shown?

Answers
Answer:
Mathematical
Explanation:
It shows how to find the hypotenuse
Brainliest
Write a ""lifeline"" of a robin over the first year of its life. Include important events.
Answers
Explanation:
Robins are born in the spring or summer and are mature birds and ready to breed in the following spring or summer. They do not mate for life. Pairs usually remain together during an entire breeding season, which can involve two or three nestings.
Since leaving the nest (fledging), it takes another 10-15 days for babies to become good fliers and individual birds. The parents continue to feed their young throughout this time.
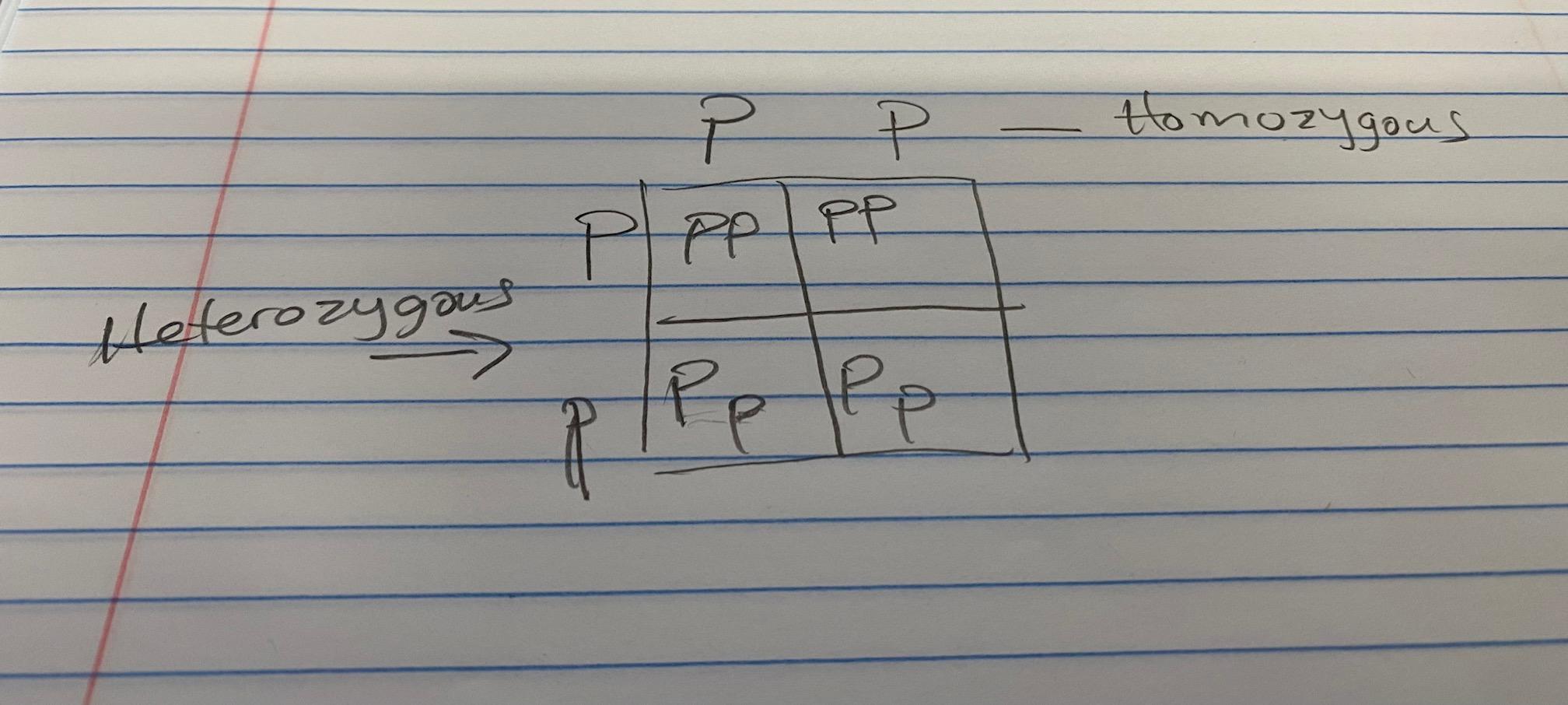
This is the picture btw because it wont let me say all the words.

Answers
Answer:
see image
D is the answer
Explanation:
see image
the box is like a mini multiplication table
What does the Law of conservation of matter say
Answers
Answer: during a chemical reaction, matter cannot be created or destroyed
Explanation:
How can a chemical reaction be sped up? Select from the drop-down menu to correctly complete the statement. Expose more of the reactant by increasing the
Answers
Answer:
I think the answer is Temperature but i don't know your options so I'm just going off of my test
Explanation:
How do geologists know that there once was a vast sea where the Grand Canyon is today?
Answers
The geologists know the presence of the vast sea at Grand Canyon by the erosion and indentations.
Grand Canyon in Arizona has been the rock sediment. It has been determined to be the vast sea due to the presence of oceanic floors. The erosion of the rocks results in the formation of the land over a vast period of time.
The rock indentation results in the formation of the mountains. The rock has been indented with the force thus, there has been the oceanic force that results in the indentations.
Thus, the erosion and indentations have resulted in the formation of the Grand Canyon by a vast sea.
For more information about the geological study, refer to the link:
https://brainly.com/question/13394878
classify each molecular art as an element or a compound.
Answers
In the given molecular art options, option B and F are elements and option A, C, D and E are compounds.
Elements refer to pure substances which are composed of only one type of atom. It refers to a class of substances that cannot be separated into simpler substances by chemical means. In the given molecular structures, option B and F are elements as they made up of single type of atoms. Compounds refer to substances which are formed by two or more different types of elements that are united chemically in fixed proportions. When the elements combine together, they react with each other and form chemical bonds as a result of sharing or exchanging electrons between atoms which are difficult to break. As molecular structures, option A, C, D and E are made up of different type of atoms, they are compound.
Learn more about Elements:
https://brainly.com/question/6258301
#SPJ4

What volume of product is produced when 20.0 Mg of liquid hydrogen reacts with
excess liquid oxygen in a rocket propellant reaction? The gas is formed at 101.325 kPa and 40˚ C
Answers
Answer: The volume of product formed is 0.26 L
Explanation:
\(\text{Moles of solute}=\frac{\text{given mass}}\times{\text{Molar Mass}}\)
\(\text{Moles of} H_2=\frac{0.02g}{2g/mol}=0.01moles\)
\(2H_2(l)+O_2(l)\rightarrow 2H_2O(g)\)
As \(O_2\) is the the excess reagent, \(H_2\) is the limiting reagent as it limits the formation of product.
According to stoichiometry :
2 moles of \(H_2\) give = 2 moles of \(H_2O\)
Thus 0.01 moles of \(H_2\) will give =\(\frac{2}{2}\times 0.01=0.01moles\) of \(H_2O\)
According to ideal gas equation:
\(PV=nRT\)
P = pressure of gas = 101.325 kPa = 1 atm
V = Volume of gas = ?
n = number of moles = 0.01
R = gas constant =\(0.0821Latm/Kmol\)
T =temperature =\(40^0C=(40+273)K=313K\)
\(V=\frac{nRT}{P}\)
\(V=\frac{0.01mol\times 0.0821L atm/K mol\times 313K}{1atm}=0.26L\)
Thus the volume of product formed is 0.26 L
Question :-
How to balance chemical equations?
I need steps
Don't spam from Go0gle
!!!!CLASS 10 CHEMISTRY!!!!
Answers
Answer:
the main aim of balance in an equation is making show that the total number of substances on the reactant side is equal to the total number of substances on the product side
Explanation:
so if we were to balance the equation of water water is a compound containing two molecules of hydrogen and one molecule of oxygen therefore
H+O=H2O
\(H+O_2= H_2O\)
Element | Reactant | Product
H | 1 × 4 | 2×2
O | 2 | 1 × 2
\(4H+O_2= 2H_2O\)
Dihydrogen monoxide is a(n) ____. A. covalent compound B. molecular formula C. empirical formula D. Ionic compound
Answers
Answer:
D. Ionic compound
Explanation:
Just based on my opinion
Correct me if I'm wrong tnx:<
Dihydrogen monoxide is a covalent compound. Option A is correct.
Covalent compounds are compounds that are held together by covalent bonds. Covalent bonds are formed when two atoms share electrons. In dihydrogen monoxide, the two hydrogen atoms share a pair of electrons with the oxygen atom. This forms a molecule of water, which is a covalent compound.
Ionic compounds are compounds that are held together by ionic bonds. Ionic bonds are formed when one atom donates electrons to another atom. In an ionic compound, the electrons are not shared, but are transferred from one atom to another. The molecular formula for dihydrogen monoxide is H₂O.
The difference between a molecular formula and an empirical formula is that the molecular formula shows the actual number of atoms of each element in a molecule, while the empirical formula shows the simplest ratio of the atoms of each element in a molecule. Option A is correct.
To know more about the Compound, here
https://brainly.com/question/29448163
#SPJ2
which flask will have the greatest number of colli- sions per second with the walls of the container?
Answers
The flask will have the greatest number of collisions per second with the walls of the container is Flask A: CO at 760 torr and 0 °C.
The Collision of the molecules with the wall of the container will be depend on the pressure. Therefore, the flask with the highest pressure have the greatest no of the collisions. As flask A has the highest pressure of 760 torr, Therefore, the flask A has the greatest number of the collusions per second with the walls of the container.
Thus the flask A with CO gas will have the greatest number of collisions per second with the walls of the container.
This question is incomplete, the complete question is :
Consider three identical flasks filled with different gases.
Flask A: CO at 760 torr and 0 °C
Flask B: N₂ at 250 torr and 0 °C
Flask C: H₂ at 100 torr and 0 °C
which flask will have the greatest number of collisions per second with the walls of the container?
To learn more about gas here
https://brainly.com/question/23056914
#SPJ4
Can someone help me pls
I'LL GIVE BRAINLEST

Answers
Answer:
its will be 4 (B) I m not sure hope that help
Answer:
i think it might be C if not im sorry pls give brainliest.. but yea im pretty sure its C
Explanation:
What is the frequency of a wave whose wavelength is 5.67 x 10^-7
Answers
The frequency of a wave whose wavelength is 5.67 × 10-⁷m is 5.3 × 10¹⁴Hz.
How to calculate frequency?Wavelength is the length of a single cycle of a wave, as measured by the distance between one peak or trough of a wave and the next.
The wavelength of a wave is often designated in physics as λ, and corresponds to the velocity of the wave divided by its frequency as follows:
λ = v/f
Where;
λ = wavelengthv = velocity of wavef = frequency of the waveThe frequency is the quotient of the number of times, n, a periodic phenomenon occurs over the time (t) in which it occurs.
According to this question, a wave has a wavelength is 5.67 × 10-⁷m. The frequency can be calculated as follows:
5.67 × 10-⁷ = 3 × 10⁸/f
f = 3 × 10⁸ ÷ 5.67 × 10-⁷
f = 5.3 × 10¹⁴Hz.
Therefore, 5.3 × 10¹⁴Hz is the frequency of the wave
Learn more about frequency of a wave at: https://brainly.com/question/14316711
#SPJ1
A solution is prepared by dissolving 0. 23 mol of nitrous acid and 0. 27 mol of sodium nitrite in water sufficient to yield 1. 00 L of solution. The addition of 0. 05 mol of HCl to this buffer solution causes the pH to drop slightly. The pH does not decrease drastically because the HCl reacts with the __________ present in the buffer solution. The Ka of nitrous acid is 1. 36 × 10-3. A) H2OB) H3O+C) nitrite ionD) nitrous acidE) This is a buffer solution: the pH does not change upon addition of acid or base
Answers
The pH does not decrease drastically because the HCl reacts with the nitrite ion present in the buffer solution is: C) nitrite ion.
What is nitrous acid (HNO2) used for?In this case, nitrous acid (HNO2) and sodium nitrite (NaNO2) are used to prepare the buffer solution. The nitrous acid is a weak acid, and the nitrite ion (NO2-) is its conjugate base. The presence of both the weak acid and its conjugate base makes this solution a buffer solution.
When a small amount of HCl is added to the buffer solution, it reacts with the nitrite ion present in the solution according to the following equation:
HCl + NO2- -> HNO2 + Cl-
The HCl donates a proton to the nitrite ion, forming nitrous acid and chloride ion. This reaction consumes some of the added HCl, which prevents the pH from decreasing drastically.
Therefore, the correct answer is option C) nitrite ion.
Learn more about nitrite ion here:https://brainly.com/question/12101971
#SPJ1
How would I justify if it’s accurate or precision

Answers
a. The measurements on the target do not represent accuracy well.
b. The measurements on the target represent precision fairly well.
What qualifies as accuracy and precision?Accuracy refers to the closeness of a measurement to the true or accepted value. Since they are off target, the measurements are not accurate.
Precision refers to the consistency and reproducibility of measurements, and in this case, the shots are clustered around certain areas, with two points in the second circle and two points in the third circle. This indicates that the person taking the shots was able to consistently hit a certain area.
Learn more on accuracy and precision here: https://brainly.com/question/1695072
#SPJ1
There are a couple sources that claim the answer is D, but I am having trouble finding out why that is.

Answers
The approximate temperature of the H₂O after thermal equilibrium was reached is 23.4°C (option d).
StepsThe amount of heat absorbed by the water is given as 300.0 J. The mass of water is 50.0 g and the specific heat capacity of water is 4.2 J/g°C. Let's use the equation:
Q = m * c * ΔT
where Q is the heat absorbed, m is the mass of the water, c is the specific heat capacity of water, and ΔT is the change in temperature.
Substituting the given values, we get:
300.0 J = 50.0 g * 4.2 J/g°C * ΔT
Solving for ΔT, we get:
ΔT = 300.0 J / (50.0 g * 4.2 J/g°C) = 1.43°C
The final temperature of the water can be calculated by adding the change in temperature to the initial temperature:
Final temperature = 22.0°C + 1.43°C ≈ 23.4°C
Therefore, the approximate temperature of the H₂O after thermal equilibrium was reached is 23.4°C (option d).
learn more about thermal equilibrium here
https://brainly.com/question/14556352
#SPJ1
and water from the masses.
2.43 g H₂O was vaporized during heating.
The molar mass of H₂O 18.02 g/mol.
How many moles of H₂O are present?
[?] mol H₂O
Keep at least one extra significant figure when reporting your
answer.
mol H₂O
Enter

Answers
The number of mole of water, H₂O present, given that 2.43 g of H₂O was vaporized is 0.13 mole
How do i determine the number of mole of H₂O present?From the above question, the following parameters were obtained:
Mass of water, H₂O = 2.43 grams Molar mass of water, H₂O = 18.02 g/mol Number of mole of water, H₂O =?Mole and mass of a substance are related by the following formula:
Mole = mass / molar mass
Inputting the given parameters, we can obtain the mole of water, H₂O as follow:
Mole of water, H₂O = 2.43/ 18.02
Mole of water, H₂O = 0.13 mole
Thus, we can say that the mole of water, H₂O present is 0.13 mole
Learn more about mole:
https://brainly.com/question/13314627
#SPJ1
as the temperature of a gas decreases is volume
Answers
Answer:
it's volume also decrease
The element iodine appears in
Period 5 of the Periodic Table. This
means that an iodine atom has-
A five electrons in its outer orbital
B five orbitals in its electron cloud
C an atomic mass greater than four
D five protons and five neutrons in its nucleus
Answers
The element iodine appears in Period 5 of the Periodic Table. This means that an iodine atom has five orbitals in its electron cloud. so the correct option is (b).
As a member of group 7, iodine possesses seven outer electrons. Since it is in the fifth period, its electrons will be in orbitals 5s and 5p. The chemical formula for iodine is 5s25px25py25pz1.
What does iodine's orbital notation mean?total amount of electrons that could occupy each orbital
Again using iodine as an example, we can observe on the periodic table that it has an atomic number of 53. ( In neutral state it contains 53 electrons). The configuration of the entire electron cloud is 1s22s22p63s23p64s23d104p65s24d105p5.
Facts about Iodine:Iodine is a trace element that can be found as a dietary supplement, as well as naturally in some foods and some forms of salt. Iodine is a necessary component of the thyroid hormones triiodothyronine (T3) and thyroxine (T4) (T3).
To know more about Iodine, visit:
https://brainly.com/question/6773013
#SPJ9
an increase in pressure increasing the billing point is an example of a-
Answers
An increase in pressure increasing the billing point is an example of a physical property.
The term "boiling point" describes the temperature at which a liquid, which is being boiled, changes from a liquid to a vapor when its pressure equals that of its environment.Physical changes are those that take place when a material's structure is the same both before and after the change.When a substance is changed into another chemical substance, this is known as chemical transformation.When describing the state of a physical system, a substance's physical qualities are those that can be measured.A substance's characteristic that is seen in a chemical reaction is referred to as a chemical property.As it demonstrates the physical state, the increase in pressure with boiling point is an example of a physical property.
Learn more about boiling point:
https://brainly.com/question/25725550
The complete question is:
An increase in pressure increasing the boiling point of a liquid is an example of a —
A physical change
B chemical change
C physical property
D chemical property
#SPJ9
How is enthalpy related to the spontaneity of a reaction?
A. AH=0 contributes to spontaneity.
B. AH<0 contributes to spontaneity.
C. AH> 0 contributes to spontaneity.
D. AH does not affect spontaneity.
Answers
Answer:
D
Explanation:
Gibb's free energy change(∆G) and Standard electrode potential of electrochemical (Ecell) determine the spontaneity of a reaction.
when ∆G > 0, the reaction is not spontaneous
∆G < 0, the reaction is spontaneous
∆G = 0, the reaction is in equilibrium
when Ecell > 0, the redox reaction is spontaneous
Ecell < 0, the redox reaction is not spontaneous
Ecell = 0, the redox reaction is in equilibrium.
ΔH < 0 contributes to spontaneity.
What is a spontaneous reaction?A spontaneous reaction is one that takes place naturally under a certain set of circumstances. The general entropy, or chaos, of the system increases in the presence of spontaneous reactions.How can we determine if a reaction is spontaneous or not?We can determine it by calculating Gibb's free energy.
How do we calculate Gibb's free energy?It is calculated by the formula:
ΔG = ΔH - TΔS
ΔG is the change in Gibb's free energy,ΔH is the change in the enthalpyΔS is the change in entropy.How does ΔG tells if a reaction is spontaneous or not?If ∆G > 0, the reaction is not a spontaneous reaction∆G < 0, the reaction is a spontaneous reaction∆G = 0, the reaction is in equilibriumHow does ΔH contribute to the spontaneity of the reaction?For a reaction to be a spontaneous reaction, ∆G < 0.Consider a reaction, ΔG will be calculated as ΔH - TΔSIf ∆H < 0 then the ΔH - TΔS will be a lesser value ( more negative) than it would have been when ∆H = 0 or ∆H > 0.Therefore ∆H < 0 contributes to spontaneity.To learn more about ΔH, spontaneous reaction, enthalpy, and entropy here,
https://brainly.com/question/12959763
#SPJ3
the difference between light cured gels is the type of photoinitiator used in the formula and the measure of _____ to which that photoinitiator responds to.
Answers
Answer: light
Explanation: done
What is the geometric shape of this molecule based on this diagram?

Answers
Explanation:
The geometric shape of this molecule is
D. tetrahedral
Hope it will help :)
Answer:
The answer will be D. tetrahedral
the osmotic pressure of an aqueous solution of 3.08 m kcl is 1.36 atm. what concentration would be necessary create an aqueous solution of ca(no3)2 with the same osmotic pressure? (assume temperature is constant)
Answers
The concentration required for the same osmotic pressure is 0.019 molL⁻¹.
The osmotic pressure of an aqueous solution is determined by the concentration of the solute particles present in the solution. To create an aqueous solution of Ca(NO₃)₂ with the same osmotic pressure as 3.08m KCl (1.36 atm), we must first determine the molarity of the solution.
The osmotic pressure can be calculated using the Van 't Hoff equation:
Osmotic Pressure (Π) = iMRT
where i is the Van 't Hoff factor (3 for Ca(NO₃)₂, as it dissociates into 3 ions), M is the molarity of the solution, R is the ideal gas constant (0.0821 L•atm•mol-1•K-1), and T is the absolute temperature (in Kelvin).
Thus, we can rearrange the equation to solve for M:
M = Π/(iRT).
Plugging in the values for Π (1.36 atm), i (3), R (0.0821 L•atm•mol⁻¹•K⁻¹), and T (298K), we get:
M = 1.36/(3*0.0821*298)
M = 0.019 molL⁻¹.
Thus, 0.019 molL⁻¹ is the molarity of the Ca(NO₃)₂ solution that would be necessary to create an aqueous solution with the same osmotic pressure of 1.36 atm as the 3.08m KCl solution.
To know more about osmotic pressure, refer here:
https://brainly.com/question/29823250#
#SPJ11
Which group (family) of non-metals does not form ions?
Answers
Answer:
Noble gasses
Explanation:
nobled gasses
all elements want to loose or gain electrons to be like the noble gasses in bonding but the noble gasses do not bond.