A technical machinist is asked to build a cubical steel tank that will hold 280 l of water. calculate in meters the smallest possible inside length of the tank. round your answer to the nearest 0.01 m
Answers
The technical machinist should build a cubical steel tank with a smallest possible inside length of 0.65 meters in order to hold 280 liters of water.
To calculate the smallest possible inside length of the cubical steel tank, we need to determine the dimensions of the tank based on its volume and the fact that it is a cube.
Given that the tank needs to hold 280 liters of water, we can convert this volume to cubic meters by dividing by 1000. So, the volume of the tank is 280/1000 = 0.28 cubic meters.
Since the tank is a cube, all sides have the same length. Let's call this length "x". Therefore, the volume of the tank can be expressed as x * x * x = x^3.
Now we can set up the equation x^3 = 0.28 and solve for x. Taking the cube root of both sides, we find x = ∛0.28.
Using a calculator, we can find the cube root of 0.28 to be approximately 0.648. This gives us the length of one side of the cube.
Rounding this value to the nearest 0.01 meter, the smallest possible inside length of the tank is 0.65 meters.
Learn more about technical machinists here:-
https://brainly.com/question/29114555
#SPJ11
Related Questions
Fission involves the _________________ of an atom into smaller particles, while fusion occurs when smaller particles _________________.
Answers
Answer:
1. Splitting
2. Combine
Explanation:
consider the following reaction: sn2 2 fe3 → sn4 2 fe2 what is the reduction half-reaction?
Answers
The reduction half-reaction in the given reaction is: 2 Fe³⁺ + 2 e⁻ → 2 Fe²⁺.
What is the half-reaction for the reduction process in the given reaction?In the given reaction, tin (Sn) undergoes a redox reaction with iron (Fe) to form tin (IV) oxide and iron (II) ions. The reduction half-reaction is the process where the iron (Fe) ions are reduced from a +3 oxidation state to a +2 oxidation state.
This is represented by the half-reaction: Fe3+ + e- → Fe2+. In this process, Fe3+ gains one electron and gets reduced to Fe2+. The oxidation number of Fe decreases from +3 to +2, indicating a reduction process. This half-reaction occurs simultaneously with the oxidation half-reaction, where Sn is oxidized from +2 to +4.
Together, these half-reactions represent the redox reaction that occurs between Sn and Fe in the given reaction.
Learn more about half-reactions
brainly.com/question/18403544
#SPJ11
Please help with this question i’ve been stuck on it!!!

Answers
Answer: 8/9
Explanation: took the quiz gang
A lump of zinc is tossed into a beaker of 500L of 14M hydrochloric acid. this reaction produces Hydrogen Gas and zinc (II) chloride. If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, what is the mass of the zinc?
Answers
If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, 2796.96 g mass of the zinc is produced .
Using the ideal gas law equation:
PV = nRT
n = (PV) / (RT)
= (1.75 atm * 645 L) / (0.0821 atm·L/(mol·K) * 400 K)
= 42.71 moles
the balanced equation for the reaction between zinc and hydrochloric acid:
Zn + 2HCl -> \(ZnCl_{2}\) + \(H_{2}\)
1 mole of zinc produces 1 mole of hydrogen gas. Therefore, the moles of zinc are also 42.71.
The molar mass of zinc is 65.38 g/mol.
Mass of zinc = moles of zinc * molar mass of zinc
= 42.71 moles * 65.38 g/mol
= 2796.96 g
Therefore, the mass of the zinc is 2796.96 grams.
learn more about hydrogen gas :
https://brainly.com/question/30829657
what is the function of digestive enzymes????
Answers
Answer:
Digestive enzymes break down food into smaller building blocks which makes it easier for absorption by the body.
Answer:
Break down the pood particles for their absorption e. g pepsin breaks down protein for its absorption in small intestine. Pepsin is present in stomach
What is the wavelength, in nm, of the light photon emitted by a hydrogen atom when an electron goes from n
Answers
Answer:
Hence, the wavelength of the photon associated is 1282 nm.
Explanation:
Which of the following is a non-volatile solute? Select all that apply.
2-propanol (rubbing alcohol)
Methanol
Sodium chloride
Sugar
Answers
the most common ion formed by oxygen is called the oxide ion. which species shows the correct charge of the oxide ion?
Answers
Peroxides species shows the correct charge of the oxide ion.
As predicted by the two partially filled outer orbitals, oxygen assumes a negative oxidation state in all of its molecules. The oxide ion O2 is produced when electron transport fills these orbitals. It is thought that each oxygen in peroxides (species that contain the ion O2 2) has a charge of 1.
The carbon and hydrogen atoms have a slight positive charge, while the oxygen atom has a slight negative charge.
There are six valence electrons in the atom of oxygen. The oxygen atom requires two additional electrons in order to complete its valence shell. Adding two more electrons gives the oxygen ion a charge of two because each electron has a single negative charge.
To know about oxide ion
https://brainly.com/question/10931958
#SPJ4
A recipe calls for 3 tablespoons of milk for 7 pancakes. If this recipe was used to make 28 pancakes, how many tablespoons of milk would be needed
A. 15
B. 11
C. 12
D. 9
Answers
The number of tablespoons of milk needed for 28 pancakes is determined as 12 tablespoons.
option C is the correct answer.
How many tablespoons of milk would be needed?The number of the tablespoons of milk that would be needed is calculated by applying simple proportion method.
3 tablespoons of milk for 7 pancakes;
3 -----------> 7
? tablespoons of milk for 28 pancakes;
? --------------------> 28
Combine the two equations and solve for the number of tablespoons needed as follows;
? = ( 3 x 28 ) / 7
? = 12
Thus, The number of tablespoons of milk needed for 28 pancakes is determined by applying simple proportion.
Learn more about proportion here: https://brainly.com/question/19994681
#SPJ1
Which would have the greatest evaporation: pure water or salt water?
Answers
Answer:
Pure Water.
Explanation:
Pure water evaporates faster than salt water.
What happens to particles of a substance as its temperature increases?
A the average kinetic energy increases
B the average kinetic energy decreases
C the average kinetic energy stays the same
D nothing happens
Answers
Answer:
A. the average kinetic energy increases
Explanation:
temperature is a measure of the average kinetic energy of the particles in a sample of matter
Pls answer. Braniest answer will be picked.

Answers
\( \huge\mathrm{ \boxed{A} \boxed{n}\boxed{s}\boxed{w}\boxed{e}\boxed{r}}\)
The Correct option is C.)
Above diagram shows how Sodium and Chlorine forms an Ionic bond.
\( \mathrm{2Na + Cl_2 \rightarrow2NaCl }\)
_____________________________
\(\mathrm{ \#TeeNForeveR}\)
the second law of thermodynamics states that disorder in a system is always increasing. in simple terms, one can think about dropping nacl crystals into a glass of water. the solvation and diffusion of ions is favored by there is an increase in: group of answer choices
Answers
Then there is an increase in Entropy.
What is entropy?A state of disorder, unpredictability, or uncertainty are frequently related with the scientific concept of entropy, which is also a quantifiable physical characteristic. The notion and the phrase are employed in a variety of disciplines, including classical thermodynamics, where they were initially identified, statistical physics, which describes nature at the molecular level, and information theory. It has wide-ranging uses in physics and chemistry, biological systems and how they relate to life, cosmology, economics, sociology, weather science, and information systems, particularly the transfer of information through telecommunication.
The amount of different configurations that the atoms in a system can take depends on its entropy. Entropy can be thought of as a gauge of uncertainty or unpredictability.
To learn more about entropy from the given link:
brainly.com/question/13146879
#SPJ4
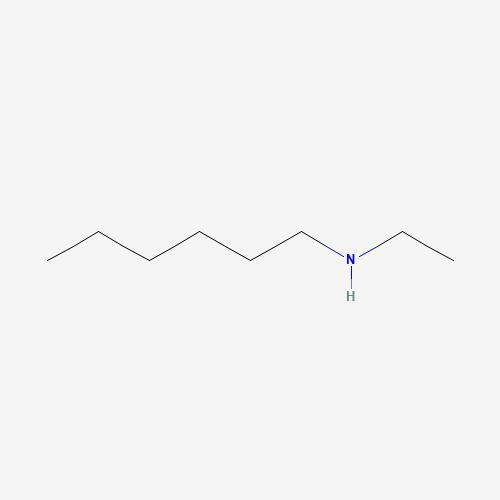
draw the structure of n-ethyl-1-hexanamine or n-ethylhexan-1-amine.
Answers
The structure of n-ethyl-1-hexanamine or n-ethylhexan-1-amine is shown in the image attached below.
N-EthylhexylamineMolecular Formula: The molecular formula for N-Ethylhexylamine is C₈H₁₉N.Synonyms: Some common synonyms for N-Ethylhexylamine are N-Ethylhexan-1-amine, 1-ethylhexylamine, and N-ethyl-1-hexylamine.Molecular Weight: The molecular weight of N-Ethylhexylamine is approximately 129.24 g/mol.Chemical Properties: N-Ethylhexylamine is a colorless to slightly yellow liquid with a strong, unpleasant odor. It is soluble in most organic solvents but has limited solubility in water. As an amine, it is a weak base, meaning it can form salts when reacting with acids. N-Ethylhexylamine has a boiling point of around 175°C and a melting point of around -69°C. It is flammable and can produce toxic fumes when burned.N-Ethylhexylamine is a versatile chemical compound used in various industries. It is used as a reagent or intermediate in chemical synthesis, a surfactant in industrial processes, a solvent in the formulation of paints, coatings, adhesives, and inks, a catalyst in certain chemical reactions, and in gas treatment processes such as removing acid gases from natural gas. It is also used as a pH regulator or stabilizer in various industrial applications.learn more about N-ethyl-1-hexanamine
https://brainly.com/question/31990221
#SPJ11

How many grams of C3H8O are in 5. 00 x 10^22 molecules?
A - 4. 99g
B -. 083g
C - 50. 0g
D - 29. 9g
Answers
The grams of the C₃H₈O are in the 5 × 10²² molecules is the A) 4.9 g
Given that :
The number of the molecules of the C₃H₈O = 5 × 10²² molecules.
The conversion of the molecules in to the moles is given below :
The number of the moles in 5 × 10²² molecules
= 5 × 10²² / 6.022 × 10²³ moles
= 0.83 × 10⁻¹ moles
The mass = moles × molar mass
The mass = 0.083 ×60
The mass = 4.9g
Thus the mass of the compound C₃H₈O are in 5 × 10²² molecules is 4.9 g.
To learn more about molecules here
https://brainly.com/question/7496559
#SPJ4
Which of the following statements is true?
A- Chemical digestion includes the physical breakdown of chunks of food.
B- Mechanical digestion begins in the mouth, and includes the tearing and grinding of food with teeth.
C- Mechanical digestion uses teeth to grind food and saliva to moisten the food
making it easier to chew.
D- Chemical digestion includes the chemical breakdown of food and mixing of the food particles with the help of the tongue
Answers
B- Mechanical digestion begins in the mouth, and includes the tearing and grinding of food with teeth.
C- Mechanical digestion uses teeth to grind food and saliva to moisten the food
making it easier to chew.
These points are true.
At what temperature would 0.0828 moles of hydrogen have a pressure of 1.00 atm and a volume of 55.0 L?
Answers
The temperature at which 0.0828 moles of hydrogen would have a pressure of 1.00 atm and a volume of 55.0 L is 743 K .
What is ideal gas law?The ideal gas law is a fundamental equation of state for an ideal gas.
We can use the Ideal Gas Law to solve for the temperature:
PV = nRT
Where
P is the pressure (in atm)V is the volume (in L)n is the number of molesR is the gas constant (0.0821 L·atm/mol·K)T is the temperature (in K)First, we need to convert the number of moles to moles:
n = 0.0828 mol
Next, we can substitute the given values into the Ideal Gas Law and solve for T:
T = PV/nR = (1.00 atm) x (55.0 L) / (0.0828 mol x 0.0821 L·atm/mol·K) = 743 K
Therefore, the temperature at which 0.0828 moles of hydrogen would have a pressure of 1.00 atm and a volume of 55.0 L is 743 K.
Learn more about ideal gas law here : brainly.com/question/27870704
#SPJ1
4,1,-1,+1/2 element in quantum numbers?
Answers
The element that has the outer shell as has been shown here is gallium.
What element has these quantum numbers?We know that the quantum numbers are the tools that can be used to be able to obtain the position of the electron in the atom. We know that it gives the description of electrons that are present in the atoms of elements.
In this case, we have the quantum numbers that describe the element as 4,1,-1,+1/2 . This shows that the electron must be in a 4p orbital. This must be an element that is in group 13 of the periodic table.
Learn more about 4p orbital:https://brainly.com/question/24258336
#SPJ1
ACELLUS LAB-ACID BASE TITRATION
a student overshot the equivalence point when he was using NaOh to determine the concentration of an unknown acid, HA. What ions are present in the purple solution in the flask?
Answers
Answer: A-, NA+ And OH-
Explanation: Thank me later
1. 86 g h2 is allowed to react with 9. 70 g n2, producing 1. 24 g nh3. Part a what is the theoretical yield in grams for this reaction under the given conditions?.
Answers
1.86 g H₂ is allowed to react with 9.70 g N₂ producing 1.24 g NH₃. the theoretical yield in grams for this reaction under given conditions is 10.54 g.
The balanced chemical equation is given by :
N₂ + 3H₂ ------> 2NH₃
it is clear from the reaction that :
1 mole of N₂ react with 3 moles of H₂ to produced 2 mole of NH₃.
the molar mass of compounds are :
H₂ = 2 g/mol
N₂ = 28 g/mol
NH₃ = 17 g/mol
3moles of H₂ × 1 g/mol= 6 g
1 mole of N₂ × 28 g/mol= 28 g
2 moles of 2NH₃ × 17 g/mol = 34 g
now we have to find out the limiting reagent :
28 g of N₂ react with 6 g of H₂ , 9.70 N₂ will react with how much mass of H₂
mass of H₂ = (9.70 g of N₂ × 6 g of H₂) / 28 g of N₂
= 2.07 g
but 2.07 g H₂ is not available , only 1.86 g are available .so, H₂ is a limiting reagent.
The theoretical yield in the given case:
mass of NH₃ = (1.86 g of H₂ × 34 g of NH₃ ) / 6 g of H₂
= 10.54 g
Thus, 1.86 g H₂ is allowed to react with 9.70 g N₂ producing 1.24 g NH₃. the theoretical yield in grams for this reaction under given conditions is 10.54 g.
To learn more about theoretical yield here
https://brainly.com/question/1379589
#SPJ1
What is conserved in the reaction shown? N_2(g)+3F_2(g)→2NF_3(g)?
Answers
The conserved in the reaction shown :
N₂(g) + 3F₂(g) ----> 2NF₃(g) is the mass is conserved.
According, to the law of conservation of mass : the mass neither be created nor be destroyed.
N₂(g) + 3F₂(g) ----> 2NF₃(g)
the mass of N₂ = 28g
the mass of 3F₂ = 114g
the mass of 2NF₃ = 142 g
N₂(g) + 3F₂(g) ----> 2NF₃(g)
28 g 114 g 142 g
28 g + 114 g = 142 g
142 g = 142 g
Thus, the mass of the reactant is equal to the mass of the product. the equation follow the law of conservation of mass as the mass of the reactant is equal to the mass of the product.
To learn more about law of conservation here
https://brainly.com/question/26078627
#SPJ4
Why does the surface of the oocyte change when the first sperm cell touches the oocyte?
Answers
To prevent more than one sperm from entering the egg.
What is oocyte?
An oocyte , or ovocyte is a female gametocyte or germ cell involved in reproduction. In other words, it is an immature ovum, or egg cell. An oocyte is produced in a female fetus in the ovary during female gametogenesis. The female germ cells produce a primordial germ cell (PGC), which then undergoes mitosis, forming oogonia. During oogenesis, the oogonia become primary oocytes. An oocyte is a form of genetic material that can be collected for cryoconservation.
When the sperm and egg fuse it triggers a release of calcium ions, which cause the cortical granules inside the egg to fuse with the plasma membrane. As they fuse, these granules release their contents outside of the cell, toward the remains of the zona pellucida.
To learn more about gametogenesis click on the link below:
https://brainly.com/question/1446790
#SPJ4
How does nuclear fusion create new elements inside of stars?A. All the nuclei repel each other because of their positive charges.B. Small nuclei cause large nuclei to break apart.C. Small nuclei combine, then form larger nuclei.
Answers
ANSWER
Small nuclei combine, then form larger nuclei.
Option C
EXPLANATION
Nuclear fusion is defined as the process in which two or more light stable nuclei combined together to form a larger nuclei
In the star region, elements are squeeze together to produce a larger one in a process called fusion. Star fuse hydrogen atoms into helium. Helium atoms then fuse to create beryllium and the process continues like that until iron is formed.
Therefore, the correct answer is option C
An average sample of lithium contains 7.5% lithium-6 and 92.5% lithium-7. Lithium-6 has an atomic mass of 6.015 amu and lithium-7 has an atomic mass of 7.016 amu. What is the average atomic mass of a sample of lithium? A B C D 6.09 amu 6.49 amu 6.52 amu 6.94 amu
Answers
The average atomic mass of Lithium is found to be 6.94 amu. Thus, (d) is the correct answer.
Atomic mass is the total number of protons and neutrons present in the nucleus of the atom when it is at rest. On comparison with protons and neutrons, electrons have negligible mass and thus atomic mass is the total sum of protons and neutrons.
Given:
% abundance of Li-6, Li6 = 7.5%
% abundance of Li-7, Li7 = 92.5%
Atomic mass of Li-6, m6 = 6.015 amu
Atomic mass of Li-7, m7 = 7.016 amu
To find:
Average atomic mass = ?
Formula:
Average atomic mass, \(Avg = \frac{(Li6 * m6) + (Li7 * m7)}{100}\)
Avg = \(\frac{(7.5 * 6.015) + (92.5 * 7.016)}{100}\)
Avg = (45.1125 + 648.98) / 100
Avg = 694.0925 / 100
Avg = 6.940 amu
Result:
Therefore, 6.94 amu is the average atomic mass of Lithium.
Learn more about atomic mass here:
https://brainly.com/question/17067547
#SPJ9
chromium (VI) phosphide
Answers
Answer:
Chromium Phosphide is a semiconductor used in high power, high frequency applications and in laser diodes.
Explanation:
The Periodic table of Elements
How many formula units are in 2.5 moles of NaCl?
Answers
Answer: 1.5 x 10 24
Explanation:
One mole of NaCl contains 6.022 x 1023 NaCl formula units
In 2.5 moles of NaCl there will be basically 5 moles of ions as per the given data.
What is a compound?A material created by joining two or more distinct elements chemically in science.
A compound in chemistry is a material comprised of two or more separate chemical elements mixed together in a certain proportion.
Chemical connections that are challenging to break are created when the elements interact with one another.
2.5 mol of NaCl contains 5 moles of ions. This is due to the fact that 1 mol of NaCl contains 2 mol of ions, so you must multiply the molecular weight of the molecule by two to obtain the number of ions.
Thus, the answer is 5 moles.
For more details regarding a compound, visit:
https://brainly.com/question/13516179
#SPJ5
Read the given list of organisms. snake, hawk, shark, leopard, wolf What best describes the role of these five organisms in a food web?
Carnivores, as they obtain food from other animals
Consumers, as they feed on either producers or meat
Herbivores, as they obtain food from plants
Consumers, as they make their own food
Answers
Answer:
Carnivore, as they obtain food from other animals
Answer:
carnivores
Explanation:
Identify one way to improve models of electron dot structure
Answers
Electron dot structures or Lewis dot structures can be improved by adding valence electrons of each atom and then representing them in form of dots.
What are Lewis dot structures?Lewis dot structures are also called as electron dot structures and can be drawn if the molecular formula of a compound is known. It provides information regarding the nature of bond and the position of atoms .
They are also capable of exhibiting the lone pair if any present in a molecule or compound.Lewis defined a base to be an electron pair donor and an acid to be an electron pair acceptor.
They are capable of reflecting electronic structure of elements and even the pairing of electrons . In the diagram, each dot represents an electron while a pair of dots represent a bond between the atoms.
Learn more about Lewis dot structures,here:
https://brainly.com/question/20300458
#SPJ1
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
(free question so use your creativity!!)
Physical science is the ____________ class in the world!
Answers
Answer:
Best
Explanation:
physical, best, greatest.