Answers
The complete equation for the precipitation reaction will be:
CuCl₂(aq)+Na₂CO₃(aq) > CuCO₃(s) + 2NaCl(aq)
From the reaction,
CuCl₂(aq)+Na₂CO₃(aq) are the reactants while CuCO₃(s) + NaCl(aq) are the products of the reaction.
In the reactants side there is 1Cu, 2Cl, 2Na and 1CO₃ while in the products side there is 1Cu, 1Cl, 1Na and 1CO₃
To balance both sides, we multiply NaCl by 2 to balance the Na and Cl atoms.
Therefore, the final balanced equation will be
CuCl₂(aq)+Na₂CO₃(aq) -----> CuCO₃(s) + 2NaCl(aq)
This reaction is balanced, and with the appropriate physical states of the reactants and products.
Read more about chemical reactions at:
brainly.com/question/29564981
#SPJ4
Related Questions
amyelkin22 Yesterday Chemistry College consider a solution containing alcohol and water. If the mole fraction of water is 0.600, what is the mole fraction of alcohol?
Answers
A solution containing alcohol and water. If the mole fraction of water is 0.600, 0.4 is the mole fraction of alcohol.
What is mole fraction?A mole fraction is a measurement of concentration that is equal to the product of the moles of a component and the total moles of the solution.
Mole fraction is indeed a unitless phrase since it represents a ratio. When all the parts of a solution's mole fraction are summed up, they equal one.
moles fraction of water + mole fraction of alcohol =1
0.600+ mole fraction of alcohol =1
mole fraction of alcohol= 1-0.600
=0.4
Therefore, 0.4 is the mole fraction of alcohol.
To know more about mole fraction, here:
https://brainly.com/question/29808190
#SPJ1
Calculate the mass, in grams, of 532.0 atoms of cadmium, Cd (1 mol of Cd has a mass of 112.41 g).
Answers
The mass, in grams, of 532.0 atoms of cadmium is 9.93 × 10⁻²⁰ g
StoichiometryFrom the question, we are to determine the mass of the given atoms of cadmium
First, we will calculate the number of moles of cadmium present
Using the formula,
\(Number\ of\ moles = \frac{Number\ of\ atoms}{Avogadro's\ constant}\)
Then,
Number of moles of Cd present = \(\frac{532.0}{6.022 \times 10^{23} }\)
Number of moles of Cd present = 8.83427 × 10⁻²² moles
From the given information,
1 mole of Cd has a mass of 112.41 g
Therefore,
8.83427 × 10⁻²² moles will have a mass of 8.83427 × 10⁻²² × 112.41 g
8.83427 × 10⁻²² × 112.41 = 9.9306 × 10⁻²⁰ g
≅ 9.93 × 10⁻²⁰ g
Hence, the mass, in grams, of 532.0 atoms of cadmium is 9.93 × 10⁻²⁰ g
Learn more on Stoichiometry here: https://brainly.com/question/13784020
Pleaseeeeeeeee
30 points
You carry out the reaction represented by the following balanced equation: N2(g) + 3H2(g) → 2NH3(g) You add an equal number of moles of nitrogen and hydrogen gases in a balloon. The volume of the balloon is 1.00 L before any reaction occurs. Determine the volume of the balloon after the reaction is complete. Assume constant temperature,
A) 0.330 L
B) 0.670 L
C) 1.00 L
D) 1.50 L
E) 3.00 L
Answers
B.
Avogadro Law was used and the stoichometric ratios and number of moles.
Thank you for this question mate. It was challenging but I hope you understand the formula.
If there's more please tell me. All the best!

The volume of the balloon can be found by making use of Avogadro's Law
and the given chemical equation.
The volume of the balloon after the reaction is B. 0.670 LReason:
The given reaction is; N₂(g) + 3H₂(g) → 2NH(g)
From the given reaction, we have;
One mole of nitrogen reacts with three moles of hydrogen to produce two
moles of ammonia.
Therefore, the reaction requires three moles of hydrogen, H₂, which is the
limiting reactant.
According to Avogadro's Law, which states that equal volumes of gas
contain equal number of molecules at the same temperature and
pressure, we have;
0.5 L of H₂ will react with \(0.5 \, L \times \frac{1}{3} = \frac{1}{6}\) L, of N₂ to produce
\(0.5 \, L \times \frac{2}{3} = \frac{1}{3}\) L of NH₃
The remaining gases in the balloon after the reaction are;
Volume of the unreacted nitrogen gas = \((\frac{1}{2} \, L - \frac{1}{6}\, L) = \frac{1}{3}\, L\) Volume of the ammonia gas produced, NH₃ = \(\frac{1}{3} L\)The volume of the balloon after the reaction is therefore;
\(\frac{1}{3} L\) of ammonia + \(\frac{1}{3}\, L\) of nitrogen gas = \(\frac{2}{3}\) ≈ 0.670 LitersThe correct option is option B. 0.670 L.
Learn more here:
https://brainly.com/question/18706897
What is the oxygen sample volume (in liters) at 65.0 ºC under these conditions?
Answers
The oxygen sample volume at 65.0 ºC and 750 mmHg pressure, containing 2.50 moles of oxygen gas, is 75.8 liters.
To determine the oxygen sample volume, we can use the Ideal Gas Law, which states that PV = nRT. To convert the temperature in Celsius to Kelvin, we add 273.15.
First, we convert the pressure from mmHg to atm by dividing by 760: 750 mmHg / 760 mmHg/atm = 0.9868 atm.
Next, we convert the temperature to Kelvin:
65.0 ºC + 273.15 = 338.15 K.
Using the given number of moles, we can rearrange the Ideal Gas Law to solve for the volume:
V = nRT/P = (2.50 mol)(0.08206 L•atm/mol•K)(338.15 K)/(0.9868 atm) = 75.8 L.
To know more about Ideal Gas Law, here
brainly.com/question/28257995
#SPJ1
--The complete Question is, What is the oxygen sample volume (in liters) at 65.0 ºC under these conditions if the pressure is 750 mmHg and the sample contains 2.50 moles of oxygen gas? --
To completely neutralise 200cm3 of 0.5mol/dm3 sodium hydroxide (NaOH), a student adds 100cm3 of 0.5mol/dm3 sulfuric acid (H2SO4). The temperature of the solution goes up by 4.5°C.
The equation for the reaction is
2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O(l)
a. Calculate the amount, in moles, of NaOH in the sodium hydroxide solution.
Answers
Answer:
0.1 mol
Explanation:
Step 1: Given data
Volume of the solution of sodium hydroxide (V): 200 cm³Molar concentration of sodium hydroxide (C): 0.5 mol/dm³Step 2: Convert "V" to dm³
We will use the conversion factor 1 dm³ = 1000 cm³.
200 cm³ × 1 dm³/1000 cm³ = 0.200 dm³
Step 3: Calculate the moles (n) of NaOH
The molarity of the NaOH solution is equal to the moles of NaOH divided by the volume of solution.
C = n/V
n = C × V
n = 0.5 mol/dm³ × 0.200 dm³ = 0.1 mol
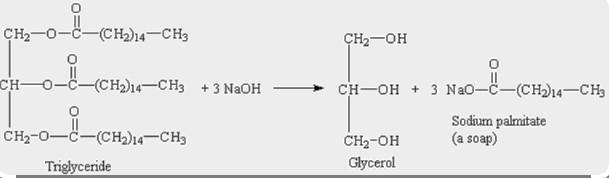
1) write a balanced equation to show the hydrolysis of glycerol tristearate (tristearin, a triple ester of glycerol) using water and sodium hydroxide to make soap. in addition to the molar ratios, give the mass ratios, i.e., the ratios of masses that react according to the reaction stoichiometry) based on 20 g of tristearin.
Answers
Mass ratio of Tristearin that react according to the reaction : \(NaOH\) is
20 : 27
The main fat in beef is tristearin. A molecule of glycerine that has interacted with three (3) molecules of the fatty acid stearic acid is known as a triglyceride.
1 mole of Tristearin requires 3 moles of \(NaOH\) to react to give 1 mole of glycerol & 3 moles of sodium sterate.
Molar ratio of reactant Tristearin : \(NaOH\) = 1 : 3
Molar weight of Tristearin = 891.5 g/mol
=> 20 g Tristearin = \(\frac{20}{891.5} moles\)
=> \(NaOH\) required to react with \(\frac{20}{891.5}\) moles of Tristearin = \(3\times\frac{20}{891.5} moles\) = 0.0673 moles
=> Molecular weight of \(NaOH\) = 40 g/mol
=> \(NaOH\) mass required to react with 20 g Tristearin = 0.0673 × 40g = 2.7g
∴ Mass ratio of Tristearin: \(NaOH\) is 20 : 27.
Learn more about Tristearin:
brainly.com/question/7175814
#SPJ4
An amateur entomologist captures a particularly excellent ladybug specimen in a plastic jar. The internal volume of the jar is 0.5L, and the air within the jar is initially at 1 atın. The bug-lover is so excited by the catch that he squeezes the jar fervently in his sweaty palm, compressing it such that the final pressure within the jar is 1.25 atm. What is the final volume of the ladybug's prison?
Answers
The final volume of the ladybug's prison is approximately 0.4 liters.
To determine the final volume of the ladybug's prison, we can use Boyle's Law, which states that the pressure and volume of a gas are inversely proportional at constant temperature. The equation for Boyle's Law is:
P1 * V1 = P2 * V2
Where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume, respectively.
In this scenario, the initial volume (V1) is given as 0.5 L, and the initial pressure (P1) is 1 atm. The final pressure (P2) is 1.25 atm. We need to find the final volume (V2).
Plugging the given values into the equation, we have:
1 atm * 0.5 L = 1.25 atm * V2
Simplifying the equation, we find:
0.5 L = 1.25 atm * V2
Dividing both sides of the equation by 1.25 atm, we get:
0.5 L / 1.25 atm = V2
V2 ≈ 0.4 L
For such more questions on volume
https://brainly.com/question/31454001
#SPJ8
Describe the advantages of the hydrogen-rich fuel cell when compared to the conventional electrochemical cells such as lead-acid battery. (4)
Answers
The hydrogen-rich fuel cell offers advantages in terms of efficiency, environmental impact, operating time, refueling speed, weight, size, and lifespan when compared to conventional electrochemical cells like the lead-acid battery.
The hydrogen-rich fuel cell offers several advantages over conventional electrochemical cells like the lead-acid battery. Here are some of the key advantages:
1. Higher Efficiency: Hydrogen fuel cells have higher energy conversion efficiencies compared to lead-acid batteries. Fuel cells can convert chemical energy directly into electrical energy with minimal loss, while lead-acid batteries have inherent energy losses due to factors such as internal resistance and heat dissipation.
2. Clean and Environmentally Friendly: Hydrogen fuel cells produce electricity through the reaction of hydrogen and oxygen, with water being the only byproduct. They do not produce harmful emissions or contribute to air pollution, making them a cleaner and more sustainable power source compared to lead-acid batteries, which require the use of chemicals like sulfuric acid.
3. Longer Operating Time: Fuel cells have longer operating times compared to lead-acid batteries. Lead-acid batteries have a limited capacity and need to be recharged frequently, while fuel cells can continuously generate electricity as long as there is a supply of hydrogen.
4. Faster Refueling: Refueling a fuel cell is faster compared to recharging a lead-acid battery. Fuel cells can be refueled by replenishing the hydrogen supply, which can be done relatively quickly. In contrast, lead-acid batteries require a longer time to recharge, typically hours, depending on the battery's capacity and charging rate.
5. Lighter Weight and Compact Size: Hydrogen fuel cells have a higher energy density compared to lead-acid batteries, meaning they can store more energy in a smaller and lighter package. This makes fuel cells more suitable for applications where weight and space are critical, such as in portable devices or electric vehicles.
6. Longer Lifespan: Fuel cells generally have a longer lifespan compared to lead-acid batteries. Lead-acid batteries can experience degradation over time due to factors like sulfation, which can reduce their overall capacity and lifespan. Fuel cells, on the other hand, can provide consistent performance over an extended period with proper maintenance.
These advantages make fuel cells a promising technology for various applications, including transportation, stationary power generation, and portable electronics.
for more questions on fuel cell
https://brainly.com/question/14122421
#SPJ8
Ga3+ and Br1- is what formula?
Answers
\(\text{GaBr}_3\)
Phthalonitrile (C₈H₄N₂) is produced by the ammoxidation of o-xylene (C₈H₁₀) according to the following reaction:
C₈H₁₀(l) + O₂(g) + NH₃(g) → C₈H₄N₂(s) + H₂O(l)
How many moles of water would be produced by the complete ammoxidation of 145.0 grams of o-xylene?
Answers
0.6066 moles of water are produced by complete ammoxidation of 145 g of o-xylene.
How are number of moles calculated?Number of moles are calculated by dividing the mass by the molar mass of the substance.In the reaction between o-xylene ,ammonia and oxygen pthalonitrile is produced.
Here, the number of o-xylene is calculated by the above mentioned formula which is 1.365 moles.Then, as per stoichiometry and balanced chemical equation ,
1.365 moles of o-xylene×8 moles of water/1 mole of xylene ×18 g water
=0.6066 moles
Thus,0.6066 moles of water are produced from 145 g of o-xylene.
Learn more about mole concept ,here:
https://brainly.com/question/22540912
#SPJ1
what are chemistry products
Answers
Answer:
Products are the species formed from chemical reactions. During a chemical reaction reactants are transformed into products after passing through a high energy transition state. This process results in the consumption of the reactants.
Explanation:
hope this helps if not let me know have a great day
write the balanced redox reaction equation?

Answers
Answer:
see the image below
Explanation:
oxidation is losing of electrons
reduction is gaining of electrons

The boiling point of another member of this homologous series was found to be 309 KK. What is the likely molecular formula for this compound?
Answers
Answer: Pentane C5H12
Explanation:
The boiling point of a substance is simply defined as the temperature whereby a liquid's vapor pressure is equal to the pressure that is surrounding the liquid and hence, the liquid will changes into vapor.
The likely molecular formula for this compound is Pentane i.e C5H12 due to the fact that its boiling point is between Butane with formula C4H10 and Hexane with formula C6H14 boiling points.
The molecular formula of the of the substance is \(\bold {C_5H_1_0}\) (pentane).
Boiling point:
It is the temperature at which liquid's vapor pressure is equal to the pressure that is surrounding the liquid and hence, the liquid start to into vaporize.
The boiling point of the given compound is 309 K which is between Butane and Hexane.
Therefore, the molecular formula of the of the substance is \(\bold {C_5H_1_0}\) (pentane).
To know more about Boiling point,
https://brainly.com/question/2153588
What could be a third quantum number of a 2p3 electron in phosphorus,
15²2s²2p63s²3p³?
A. m/= -1
OB. m/= 2
OC. m₁= 3
OD. m/= -2
Answers
The third quantum number of a 2p\(^{3}\) electron in phosphorus is ml= -1.
Quantum numbers are the set of numbers describe the position of electron and energy of electron in an atom.there are four types of quantum numbers.
Electronic configuration of phosphorus is 1s\(^{2}\) 2s\(^{2}\) 2p\(^{6}\) 3s\(^{2}\) 3p\(^{6}\)
Quantum numbers for 2p\(^{3}\)
1) Principal quantum number (n) = 2
2) Azimuthal quantum number (l) = (n-1) = 1
3) magnetic quantum number (ml) = -l to +l = -1 , 0 , +1
form the given option , ml = -1 for n= 2 , l = 1
Thus,The third quantum number of a 2p\(^{3}\) electron in phosphorus is m= -1
To learn more about Quantum numbers here
https://brainly.com/question/25117259
#SPJ1
Answer: A. m/= -1
Explanation:

What might happen if water molecules did not have a slight negative charge on one end and a slight positive charge on another
Answers
Water molecules did not have a slight negative charge on one end and a slight positive charge on another, the loss of polarity would have profound effects on various biological, chemical, and physical processes. The unique properties of water that are vital for life as we know it would be significantly altered, potentially rendering many biological systems nonfunctional and disrupting the stability of ecosystems.
Loss of hydrogen bonding: The polarity of water molecules allows them to form hydrogen bonds with each other and with other polar substances.Hydrogen bonds are relatively weak but essential for various biological processes, including protein folding, DNA structure, and the stabilization of cell membranes. Altered solubility: Water's polarity contributes to its excellent solvent properties. It can dissolve a wide range of substances, including salts, sugars, and polar molecules, due to its ability to surround and separate charged or polar particles. Changes in boiling and freezing points: The polarity of water affects its boiling and freezing points. Water has a relatively high boiling point and melting point compared to other substances of similar molecular weight. Altered surface tension: Surface tension is the cohesive force that holds the surface of a liquid together. Water exhibits relatively high surface tension due to the cohesive forces between water molecules resulting from their polarity. Changes in heat capacity: Water's ability to absorb and retain heat is crucial for temperature regulation in many organisms and helps moderate temperature changes in the environment.For such more question on Water molecules
https://brainly.com/question/21426318
#SPJ8
Find the mass in grams of 4.60 x 10^23 atoms
Answers
The mass in grams of 4.60 x 10^23 atoms is approximately 9.17 g.
To find the mass in grams of 4.60 x 10^23 atoms, we need to consider the molar mass and Avogadro's number. Avogadro's number (6.022 x 10^23) represents the number of atoms or molecules in one mole of a substance.
First, we need to determine the molar mass of the substance in question. Let's assume we are dealing with a specific element, such as carbon (C), which has a molar mass of approximately 12.01 g/mol.
To calculate the mass in grams, we can use the following formula:
Mass (in grams) = (Number of atoms / Avogadro's number) x Molar mass
Substituting the given values:
Mass (in grams) = (4.60 x 10^23 atoms / 6.022 x 10^23) x 12.01 g/mol
Calculating the expression:
Mass (in grams) = (0.763 mol) x 12.01 g/mol
Mass (in grams) = 9.17 g
For such more questions on atoms
https://brainly.com/question/6258301
#SPJ8
1
Which of the following is an example of a nonrenewable
resource?
A
B
C
D

Answers
Answer:
B not really sure tho :)
Explanation:
helppp, its equation balancing!
the answer is C but how?

Answers
Answer:
12
Explanation:
it's because the question is ,,,the balance of a and c if u count the letter a its equal to 14 then if u count the letter c its equal to 10 so the letter a give to letter c a 2 that's why we can balance into 12 the letter a become 12 and letter c become 12 also.
Can the question, Should industries releasing heavy metals into land and water ecosystems be penalized? Be answered by science or not?
Answers
Answer:
\(No\)Explanation:
Here, we want to get if the presented question could be answered by science or not
Looking at the question, we can see it has to do with legislation. The underlying question of if the substances released are harmful could be answered by science. However, after determining the harmful nature of these releases. The required legislation is something that science has nothing to do with
Thus, we can see penalization after discovery of the harmful nature is not a question for the sciences
What would be the kinetic energy, in J, of an electron with a wavelength of 0.445 nm, which would be equivalent to the wavelength of electromagnetic radiation in the X-ray region? (The mass of an electron is 9.11 × 10⁻²⁸ g.)
Answers
Answer:
The kinetic energy of the electron is approximately 4.45 × 10^-15 J, assuming that the electron is moving at a velocity of about 1.198 × 10^7 m/s.
Explanation:
We can use the formula for the energy of a photon of electromagnetic radiation:
E = hc/λ
where h is Planck's constant (6.626 × 10^-34 J·s), c is the speed of light (2.998 × 10^8 m/s), and λ is the wavelength of the radiation.
Since the wavelength of the electron in this question is equivalent to the wavelength of X-ray radiation, we can assume that the energy of the electron is equal to the energy of a photon of X-ray radiation with the same wavelength.
So, we can calculate the energy of the photon:
E = hc/λ = (6.626 × 10^-34 J·s × 2.998 × 10^8 m/s)/(0.445 × 10^-9 m) ≈ 4.45 × 10^-15 J
Since the electron has the same energy as the photon, its kinetic energy is also approximately 4.45 × 10^-15 J.
To convert the mass of the electron from grams to kilograms, we divide by 1000:
mass of electron = 9.11 × 10^-28 kg
Using the formula for kinetic energy:
KE = (1/2)mv^2
where m is the mass of the electron and v is its velocity, we can solve for the velocity of the electron:
KE = (1/2)mv^2
v^2 = (2KE)/m
v = √((2KE)/m)
Substituting the values we have calculated, we get:
√((2KE)/m) = √((2 × 4.45 × 10^-15 J)/(9.11 × 10^-28 kg)) ≈ 1.198 × 10^7 m/s
If the caffeine concentration in a particular brand of soda is 2.99 mg/oz, drinking how many cans of soda would be lethal? Assume 10.0 grams of caffeine is a lethal dose, and they are 12 oz in a can
Answers
If the caffeine concentration in a particular brand of soda is 2.99 mg/oz, drinking, The number of cans of soda would be lethal is 258 cans.
What is caffeine ?Caffeine is a stimulant. In the brain, it blocks the effects of a chemical called adenosine, which makes you feel sleepy. we then feel more alert and energetic, which is why many people drink soda, coffee or tea to stay awake. Caffeine may keep you awake even if you don't want it to
Given
1000 mg = 1 g10.0 g= 10 000 mgv = 10 000/3.23 =3095.96 oz
Therefore,
Number of cans = 3095.96 /12 =258 cans
If the caffeine concentration in a particular brand of soda is 2.99 mg/oz, drinking, The number of cans of soda would be lethal is 258 cans.
Learn more about Caffeine here ;
https://brainly.com/question/2677136
#SPJ1
What is the frequency of light that has a wavelength of 375 nm?
Answers
Answer:
f = 8×10¹⁴ s
Explanation:
λ × f = c ⇒ f = c/λ
(c = speed of light [3×10⁸ m/s])
(λ = wavelength)
1. Convert 375 nm to meters in order to multiply by speed of light unit (m/s)
375 nm × \(\frac{1 meter}{10 ^{9} nm}\) = 3.75 × 10⁻⁷ m
2. Plug in converted wavelength to top equation
f = (3×10⁸ m/s)/(3.75 × 10⁻⁷ m)
f = 8×10¹⁴ s
Which of the following conclusions can be drawn from the curves in the
energy diagram?
OA. The reaction represented by curve B will go faster than the curve A
reaction.
OB. The molecules represented in curve A have higher kinetic energy
than curve B.
C. The reaction represented by curve B has a larger equilibrium
constant than curve A.
OD. The reaction represented by curve A requires less added energy
than curve B.
SUBMIT
Answers
OD. The reaction represented by curve A requires less added energy than curve B.
Curve A represents an exothermic reaction because the products have less energy than the reactants, and curve B represents an endothermic reaction because the products have more energy than the reactants. The difference in energy between the reactants and products in curve A is less than the difference in energy between the reactants and products in curve B, which means that less energy is required to reach the transition state in curve A than in curve B. Therefore, curve A requires less added energy than curve B.
Option A is incorrect because the speed of a reaction is not determined by the shape of the energy diagram.
Option B is incorrect because the shape of the curves does not provide information about the kinetic energy of the molecules.
Option C is incorrect because the size of the equilibrium constant is not determined by the shape of the energy diagram.
which isotope has 30 neutrons and 32 protons
Answers
Answer:
Germanium
Explanation:
A Question 2 (2 points) Retake question
Potassium chlorate decomposes into potassium chloride and oxygen gas according to
the following equation:
2 KClO3 --> 2 KCl + 3 02
Use the equation above to answer the questions that follow:
How many moles of oxygen will be produced from the decomposition of 276 g of
potassium chlorate?
Do not put the answer in scientific notation. Do not include the substance as part of
your unit. Units should be all lowercase letters and singular (not plural) and no
spaces among units. You will choose from the following units:

Answers
Answer:
3.3765 Mol O2
Explanation:
There is no work for this problem
write the balanced equation for the standard molar enthalphy change formation of ethanol
Answers
Ethanol is formed by the combination of carbon, hydrogen and oxygen as shown in the image attached.
What is ethanol?We know that ethanol is an organic compound and the compound is part of the alkanol family of the organic compounds. In this case we are trying to find the equation that shows the enthalpy of the formation of the ethanol.
We know that such equation would have to involve the pure substances that can be combined so as to form the ethanol and the equation for this reaction has been shown in the image attached to this answer.
Learn more about reaction:https://brainly.com/question/28984750
#SPJ1

Use your understanding of the ideal gas law to
identify the correct relationships among the
variables.
Pressure is
Temperature is
Volume is
Moles are
Answers
The ideal gas relationships among the variables are PV = nRT
An ideal gas is a theoretical gas composed of many randomly transferring factor particles that aren't difficult to interparticle interactions. the best gasoline idea is beneficial because it obeys the precise gas law, a simplified equation of country, and is amenable to evaluation under statistical mechanics.
Volume is a degree of occupied three-dimensional space. it's far more frequently quantified numerically the usage of SI-derived gadgets or by way of diverse imperial gadgets. The definition of length is interrelated with the extent.
An ideal gas is described as one for which both the extent of molecules and forces between the molecules are so small that they have got no effect on the behavior of the gas. The real gas that acts almost like a really perfect gasoline is helium. that is due to the fact helium, in contrast to maximum gases, exists as an unmarried atom, which makes the van der Waals dispersion forces as low as viable
relation
PV = nRT
Learn more about ideal gas here:-https://brainly.com/question/20348074
#SPJ1
What does the number 84 in the name krypton-84 represent?
a) the atomic number
b) the mass number
c) the sum of the protons and electrons
d) twice the number of protons
Answers
Answer:
b
Explanation:
it express the mass no of the element
HELP PLEASE PLEASE PLEASE. Can anyone tell me how to separate the following mixture
A) ethanol in water
B) boiling the mixture of chloride crystals with water
C) pure water from muddy water
D) sodium chloride in water
E) sodium carbonate in water
F) chlorophyll from leaves
G) mixture of acetic acid and alcohol
H) serum from blood sample
I) kerosene from water
J) ammonium chloride in sand
I NEED CORRECT ANSWERS ONLY.
HURRY UP PLEASE. I WILL MARK AS BRAINLIEST
Answers
A) Ethanol in water: Distillation.
B) Boiling the mixture of chloride crystals with water: Evaporation.
C) Pure water from muddy water: Filtration.
D) Sodium chloride in water: Evaporation or Crystallization.
E) Sodium carbonate in water: Filtration or Evaporation.
F) Chlorophyll from leaves: Extraction using a suitable solvent like ethanol.
G) Mixture of acetic acid and alcohol: Distillation.
H) Serum from blood sample: Centrifugation.
I) Kerosene from water: Separatory funnel or Decantation.
J) Ammonium chloride in sand: Sublimation or Dissolving in water and Filtration.
A) Ethanol in water: Distillation can be used to separate ethanol from water based on their different boiling points.
B) Boiling the mixture of chloride crystals with water: By heating the mixture, the water will evaporate, leaving behind the chloride crystals.
C) Pure water from muddy water: Filtration can be used to separate the solid particles (mud) from the water.
D) Sodium chloride in water: Evaporation can be used to separate sodium chloride from water by heating the mixture until the water evaporates, leaving behind the salt.
E) Sodium carbonate in water: Filtration can be used to separate solid sodium carbonate from water, similar to muddy water.
F) Chlorophyll from leaves: Extraction using a suitable solvent like ethanol or acetone can be used to separate chlorophyll from leaves.
G) Mixture of acetic acid and alcohol: Distillation can be used to separate the mixture based on their different boiling points.
H) Serum from blood sample: Centrifugation can be used to separate the serum, which is the liquid part of blood, from the solid components like cells.
I) Kerosene from water: Separatory funnel or decantation can be used to separate the immiscible liquids by pouring off the top layer (kerosene) from the bottom layer (water).
J) Ammonium chloride in sand: Sublimation can be used to separate ammonium chloride by heating the mixture, causing the ammonium chloride to vaporize and then condense back into solid form in a cooler region, leaving the sand behind.
Know more about Sublimation here:
https://brainly.com/question/16789108
#SPJ8
Heat energy from the sun heats water on earth’s surface. The heated water evaporates and the water vapor rises into the air. As the water vapor rises, it cools and condenses around dust particles, and returns to earth’s surface as precipitation. This is a
A.
the water cycle
B.
the oxygen cycle
C.
the heat cycle
D.
the carbon cycle
Answers
Answer:
A - The water cycle
Explanation:
Condensation is the process by which water vapor in the air is changed into liquid water. These clouds may produce precipitation, which is the primary route for water to return to the Earth's surface within the water cycle. Condensation is the opposite of evaporation.