Does Q for the formation of 1 mol of NH₃ from H₂ and N₂ differ from Q for the formation of NH₃ from H₂ and 1 mol of N₂? Explain and give the relationship between the two Q’s.
Answers
Yes, the formation of 1 mol of NH3 from H2 and N2 differ from Q for the formation of NH3 from H2 and 1mol of N2
Equation of the formation of NH3
3/2 H2 (g)+ 1/2 N2 (g) → NH3 (g)
The Q of this reaction is
Q1 = [product] / [reactant]
Q1 = [NH 3 ] / [H2]^3/2 [N2]^1/2
Equation of the formation of NO3
3H 2 (g) + N2(g) → 2NH 3(g)
The Q of this reaction
Q 2 = [product] / [reactant]
Q 2 = [NH 3 ]^2 / [H 2 ]^3 [N 2 ]
The relationship between the two Q's is
(Q1)^2 = Q2
The reaction percentage Q is a metric for the proportions of products and reactants in a reaction at a specific moment. Because Q is a concept strongly related to the equilibrium constant K, this formula might look strangely familiar to you.
To learn more about the Q click the link
https://brainly.com/question/12693045
#SPJ4
Related Questions
I WILL GIVE YOU BRAINLIEST HELP ME!!!
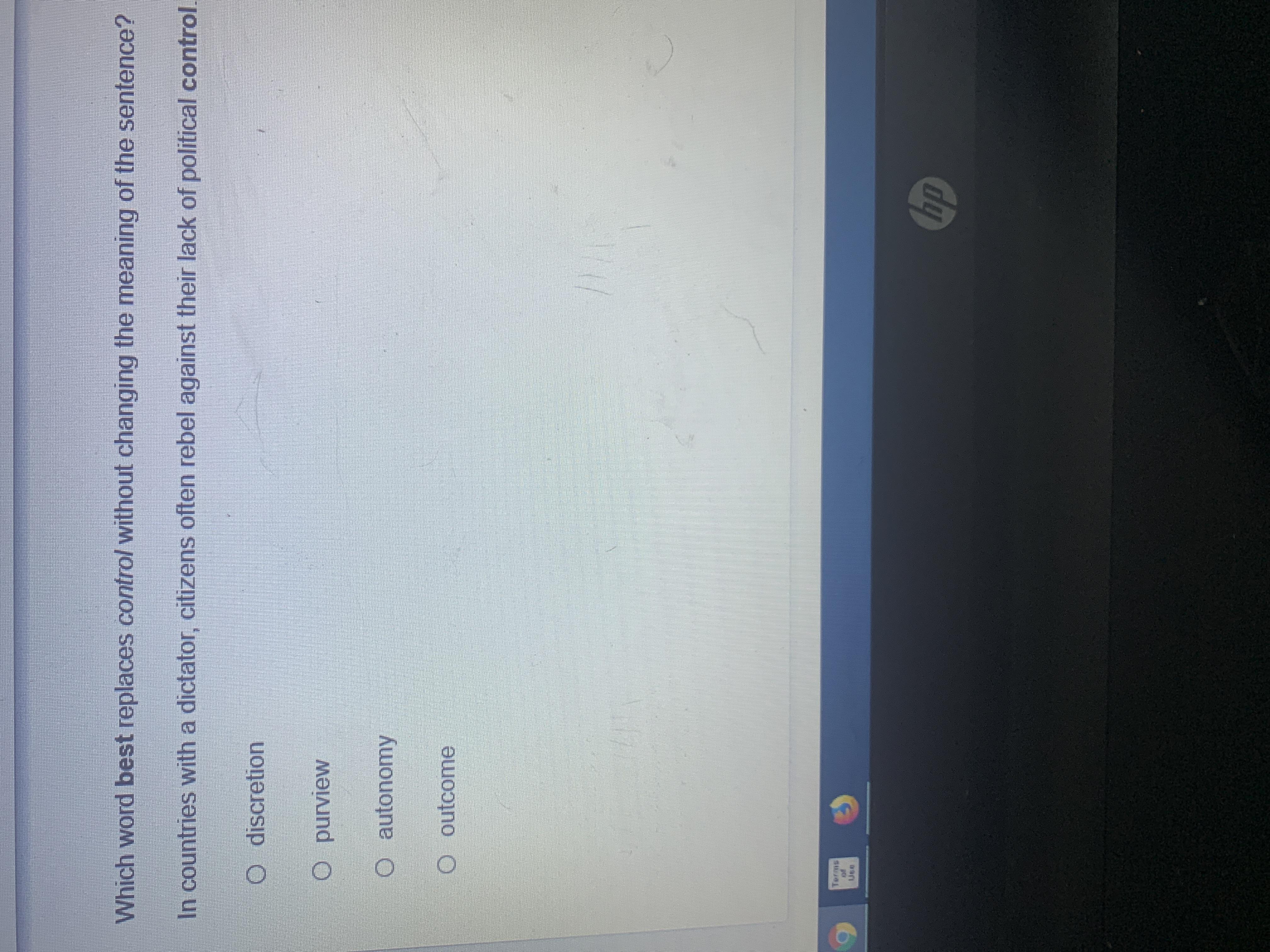
Answers
Answer:
It is the first one
Explanation:
Due to the fact that political discretion would be so only a few or a group of indviduals could control or know certain things
Answer: yeah is the first one
Explanation:
The equilibrium constant of a reaction requires certain environmental variables to remain constant. These variables are _____.
pressure, temperature, and concentration
temperature and concentration
pressure, temperature, and time
None of the above.
Answers
The equilibrium constant of a reaction requires certain environmental variables to remain constant. These variables are pressure, temperature, and concentration. The correct option is A.
An equilibrium constant is a mathematical tool that enables the quantification of the extent of a chemical reaction. The equilibrium constant is symbolized by Keq, and it is utilized to determine the concentration of reactants and products present at equilibrium.
This calculation is done using the law of mass action.Keq is defined as the ratio of product concentrations to reactant concentrations in a chemical reaction taking place at equilibrium. The concentrations used in the expression for Keq are equilibrium concentrations.
As a result, Keq is a constant for a given reaction at a specific temperature. Keq is dependent on a variety of environmental variables such as temperature, pressure, and concentration. To keep the equilibrium constant stable, these variables must remain constant.
Learn more about equilibrium constant
brainly.com/question/28559466
#SPJ11
The peptide bond between which two amino acid residues is cleaved by hiv protease?
Answers
The peptide bond between which two amino acid residues is cleaved by HIV protease will be "Phenylalanine Proline".
Whenever the carboxyl group with one molecule combines at all with the amino group of the other molecule, a molecule of water is released, and a peptide bond is created among the two molecules (\(H_{2} O\))
The HIV protease breaks down large precursor proteins towards smaller ones. A new HIV virus is created when these smaller proteins interact with both the genetic material of HIV. HIV cannot replicate when protease is blocked by protease inhibitors (PIs).
HIV protease breaks down freshly created polyproteins specifically, Gag as well as Gag-Pol at nine cleavage sites to produce the mature protein components of such an HIV virion, the infectious version of the virus beyond the host cell. HIV virions do not spread disease in the absence of an efficient HIV protease.
Therefore, the peptide bond between which two amino acid residues is cleaved by HIV protease will be "Phenylalanine Proline".
To know more about peptide bond
https://brainly.com/question/18881442
#SPJ4
Balance this reaction:
C6H10Cl4+Cl2=
Answers
Answer:
C6H10 + 2Cl2 → C6H10Cl4
Explanation:
we count atoms by looking at the
Answers
Please help, would be greatly appreciated!

Answers
According to the stoichiometry of the given chemical equation 102.03 g of Al₂O₃ is formed from 54 g of aluminium.
What is stoichiometry?It is the determination of proportions of elements or compounds in a chemical reaction. The related relations are based on law of conservation of mass and law of combining weights and volumes.
Stoichiometry is used in quantitative analysis for measuring concentrations of substances present in the sample. As per stoichiometry of the given chemical equation, 107.92 g aluminium gives 203.92 g Al₂O₃, thus 54 g aluminium will give 54×203.92/107.92=102.03 g.
Thus, 102.03 g of Al₂O₃ is formed from 54 g of aluminium.
Learn more about stoichiometry,here:
https://brainly.com/question/9743981
#SPJ1
All matter has both physical and chemical properties. A physical property can be observed without changing the identity of the substance. Which is a physical property?
A. ability to rust
B. density
C. flammability
D. reactivity with the water
Answers
Explanation:
Density
Hope it helps :)
What is EP measured in physics?.
Answers
Ep = mgh. energy associated with gravity. Ep = mgh. The labor required to lift an object is equal to the gravitational potential energy it contains.
What is the best way to define potential energy?Potential energy is simply stored energy that has the capacity to perform work as a result of the location or state of the object in question. Potential energy is described as the energy held within a system of physically interfering entities in more physics-focused terminology.
Can potential energy be negative?Because of where you choose to establish your zero point—the location at which your potential energy is zero—potential energy may also be negative. A book on a table has more potential energy than the floor, which is the potential energy zero.
To know more about potential energy visit:
https://brainly.com/question/24284560
#SPJ4
do eukaryotic Cells have DNA found in the nucleus. True or false
Answers
100 PIONTS PLZZZ HELP!!!!!
Determine whether each of the following will undergo dissociation,
dissolving or ionization. Then write the solvation equation for each
substance:
a) Lithium oxide
b) Magnesium nitride
c) Hydrobromic acid
Answers
Answer:
The answer is on the image.
Hope it helps!

Answer:Lithium oxide is able to undergo both the processes of dissolution and dissociation.
Dissociation refers to the break down a chemical substance into its constituents. Ionization refers to the loss of electrons from a chemical specie while dissolving refers to the ability of a solid to form a solution.
Let us now look at each of the following species;
Lithium oxide undergoes dissolution and dissociation.
Magnesium nitride undergoes dissolution and dissociation.
Hydrobromic acid undergoes dissolution and dissociation.
Explanation:
Extensive properties _____. ado not depend on the amount of matter present binclude the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid and the liquid changes into a vapor call depend on the chemical composition of the matter present dinclude the quantity of three-dimensional space enclosed by some closed boundary as well as mass
Answers
Extensive properties include the quantity of three-dimensional space enclosed by some closed boundary as well as mass.
What do you mean by extensive property?
A property of matter that alters as the amount of the substance changes is called an extensive property. An extensive property, like other physical qualities, can be seen and measured without any chemical change (reaction) taking place.
Intensive vs. Extensive PropertiesUnlike extensive characteristics, intensive properties are independent of the volume of the sample. No matter how much material you are looking at—large or small—they are all the same. Electrical conductivity is one such intense feature. A wire's composition, not its length, determines how electrically conductive it is. Two other illustrations of intense attributes are density and solubility.
Learn more about the intensive property here:-
https://brainly.com/question/1496355
#SPJ4
The bond breaking process has ∆H (greater, less) than zero and ∆S (greater, less) than zero; thus it is favorable at (high, low) tem- peratures.
1. less; less; high.
2. greater; less; high.
3. less; less; low.
4. less; greater; high.
5. greater; greater; low. 6. less; greater; low.
7. greater; less; low.
8. greater; greater; high.
Answers
The bond breaking process has ∆H less than zero and ∆S les than zero; thus it is favorable at low temperatures, option 3.
The bond breaking process requires energy input, which means it has a positive ∆H value. Additionally, breaking bonds usually leads to an increase in disorder, so ∆S is also positive. However, at low temperatures, the positive ∆H term dominates and the process becomes unfavorable.
Therefore, the bond breaking process is favorable at high temperatures, where the positive ∆S term becomes more significant and can offset the positive ∆H term.
Therefore, correct answer is option 3
To know more about bond breaking process click on below link :
https://brainly.com/question/29168440#
#SPJ11
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
Convert 48.5 grams of water to moles of water
Answers
Answer:
2.70 mol of water.
Explanation:
divide the given mass by the molar mass of water. 48.5 / 18.016g = 2.70 mol water.
For the reaction CH3COOH→CH3COO– + H+, which statement is true?
CH3COOH is a Brønsted-Lowry base.
CH3COO– is an Arrhenius base.
CH3COOH is a Brønsted-Lowry acid.
CH3COO– is a Lewis base.
Answers
Answer:
For the reaction CH3COOH→CH3COO– + H+
Among the given statements which is the correct statement?
CH3COOH is a Brønsted-Lowry base.
CH3COO– is an Arrhenius base.
CH3COOH is a Brønsted-Lowry acid.
CH3COO– is a Lewis base.
Explanation:
Bronsted acid is the proton donor.
Bronsted base is the proton acceptor.
Arrhenius base is the one that releases OH- ions when dissolved in water.
Lewis base is the one that is an electron-pair donor.
In the given reaction,
CH3COOH→CH3COO– + H+
Acetic acid releases a proton.
So, it is a proton donor.
Hence, It is a Bronsted-Lowry acid.
Third option is the correct answer.
Which of the following is the smallest subatomic particle in an atom?
A: Neutron
B: Proton
C: Electron
D: Compound
Answers
Answer:electron
Explanation:
12. Which of these elements is most likely to attract electrons from another atom during chemical bonding?
O 1) Fe
2) C
3) AI
4) Cs
Answers
I think the correct answer of these qusion is 2
During a reaction, the enthalpy of formation of an intermediate is 90.3 kJ/mol. During the reaction, 2 moles of the intermediate are formed as a reactant. What is the enthalpy value for this step of the reaction?
A. -180.6 kJ
B. 90.3 kJ
C. -90.3 kJ
D. 180.6 kJ
Answers
Answer:
180.6 kJ
Explanation:
The enthalpy of reaction refers to the energy absorbed or released during a reaction. If heat is absorbed in a reaction, the enthalpy of reaction is positive. If heat is released in a reaction, the enthalpy of reaction his negative.
Since the energy absorbed when one mole of the intermediate is formed is 90.3 kJ/mol, then, when two moles of intermediate is formed, 2 × 90.3 kJ/mol = 180.6 kJ of energy is absorbed.
What is the molarity of 20g of KNO, in 250 mL of water?
Answers
Answer:
should be 2M
Explanation:
:)
Which item can only be observed using some kind of instrument?
1.heat from the Sun
2.the planet Venus
3.a radio signal
4.an earthquake
Answers
Which agricultural practices result in methane emission? Select the two correct answers.a. clearing land for farmsb. refrigerationc. manure management techniquesd. rice cultivation
Answers
The agricultural practices that results in methane emission is manure management. That is option C.
What is methane gas emission?Methane gas emission is defined as the release of large quantities of methane gas into the atmospheric region of the earth by production and transport of coal, natural gas, and oil, livestock and other agricultural practices, land use and by the decay of organic waste in municipal solid waste landfills.
The negative effects of the continuous emission of methane gas into the atmosphere would lead to global warming as it is one of the major greenhouse gases.
Manure management is one of the agricultural practices that can lead to the emission of methane gases.
Learn more about gases here:
https://brainly.com/question/25736513
#SPJ1
What best describes the interaction described below:
Dodder and shrub. The Dodder plant grows on the shrub. The dodder takes water from the shrub and the branches of the dodder pierce the tissues of the shrub. Eventually the shrub will die.
Group of answer choices
Answers
One method of obtaining these nutrients from the shrub is through the branches of the Dodder plant penetrating the tissues of the shrub. This is an illustration of a partnership in which only one creature benefits.
What really is tissue and what does it do?Tissue is a collection of cells with a common structure and function that work as a single unit. The brain give it shape and help it retain heat and energy. ibrocartilage, epithelial tissue, muscle tissue, and nervous tissue are the four different types of tissues.
What do cells in tissue do?Your organism is made up of cells, and tissues are created when groupings of cells carry out similar tasks. Your body consists mostly of four different tissue types: connective, epithelial, muscular, and nervous tissue. Organs are cushioned and joined together by connective tissue. The skin's outer layer is made up of epithelial tissue.
To know more about tissues visit:
https://brainly.com/question/17664886
#SPJ1
A stock solution of sodium sulfate, Na2SO4 has a concentration of 1.00 M. The volume of this solution is 50 mL. What volume of a 0.25 M solution could be made from the stock solution?
Answers
We can make a 0.25 M solution with a volume of 200 mL from the stock solution.
How do you calculate the volume of a 0.25 M solution that could be made from the stock solution?We can use the dilution formula to fix this issue:
C1V1 = C2V2
where C1 and V1 represent the initial (stock) solution's concentration and volume, and C2 and V2 represent the end (diluted) solution's concentration and volume.
In order to write: "We want to determine the volume of a 0.25 M solution that can be created from the stock solution."
C2 = 0.25 M V2 =? where C1 = 1.00 M and V1 = 50 mL.
Using the dilution formula with these values as input, we obtain:
0.25 M x V2 = 1.00 M x 50 mL
If we simplify, we get:
50 mL = 0.25 M x V2
The result of dividing both sides by 0.25 M is:
V2 = 200 mL
To learn more about stock solution visit:
brainly.com/question/25256765
#SPJ1
Calcium carbide, CaC₂ reacts with water to form ethyne, C₂H₂, and calcium hydroxide.
the equation for the reaction is shown.
CaC₂(s) + 2H₂O(l)→ C₂H₂(g) + Ca(OH)2(s)
which Volume of ethyne is produced when 6g of water react completely with calcium carbide?
A. 4dm^3
B. 8dm^3
C. 36dm^3
D. 72dm^3
( please solve explanation!! )
Answers
Answer:
36dm³
Explanation:
For this question we are required to find the volume of the ethyne which is released while reacting 6 grams of water with calcium carbide.
Here to find the volume of ethyne produced, we can find the moles of the ethyne in the reaction by using the mole concept, and then converting the mol into the volume using the formula V=nRT/p or PV=nRT (n being the moles of the gas)
as a puck falls its potential energy decreases why
Answers
Answer:
because its getting converted into another form of energy, as the puck is falling its increasing its velocity at a rate which decreases its potential energy and increases its frictional energy which then converts to kinetic energy as it hits an object or the floor.
Explanation:
what is the theoretical yield, in grams, of co2 , if 27.4 g of c2h2 completely reacts?
Answers
The theoretical yield of \(CO_2\) is 92.58 grams when 27.4 grams of \(C_2H_2\) completely reacts.
To calculate the theoretical yield of \(CO_2\) in grams when 27.4 g of \(C_2H_2\) completely reacts, follow these steps:
1. Write the balanced chemical equation for the reaction:
\(C_2H_2\) + (5/2) \(O_2\) -> 2 \(CO_2\) + \(H_2O\)
2. Determine the molar mass of \(C_2H_2\) and \(CO_2\) :
\(C_2H_2\): 2(12.01 g/mol) + 2(1.01 g/mol) = 26.04 g/mol
\(CO_2\) : 12.01 g/mol + 2(16.00 g/mol) = 44.01 g/mol
3. Calculate the moles of \(C_2H_2\):
moles = (mass of \(C_2H_2\)) / (molar mass of \(C_2H_2\)) = 27.4 g / 26.04 g/mol = 1.052 moles
4. Use the stoichiometry of the balanced equation to determine moles of \(CO_2\) produced:
1 mole of \(C_2H_2\) produces 2 moles of \(CO_2\), so 1.052 moles of \(C_2H_2\) will produce 2 * 1.052 = 2.104 moles of \(CO_2\).
5. Calculate the mass of \(CO_2\) produced:
mass = (moles of \(CO_2\) ) * (molar mass of \(CO_2\) ) = 2.104 moles * 44.01 g/mol = 92.58 g
So, the theoretical yield of \(CO_2\) is 92.58 grams when 27.4 grams of \(C_2H_2\) completely reacts.
To learn more about theoretical yield refer: https://brainly.com/question/30808216
#SPJ11
please help
1. State the relationship between atomic radius and metallic reactivity down group 2. Explain in terms of atomic structure why this relationship occurs. as atomic radius down group 2
Answers
Answer:
Atomic radius is directly proportional to Metallic reactivity in group 2.
Explanation:
Atomic radius is directly proportional to Metallic reactivity in group 2.
As we move down the group in group 2 which is called as the alkaline earth metals, the atomic number increases due to which the subshell increases. And the outermost shell's electron are less tightly held to the nucleus of the atom. Since the electrons are loosely held that means they can easily participate in reactions and that what makes it reactive down the 2nd group. Therefore alkaline earth metals with higher atomic number are more chemically reactive.
How much agco is formed when 25.0 ml of 0.200 m agno is mixed with 50.0 ml of 0.800 m naco?
Answers
The Ag\(_{2}\)CO\(_{3}\) is formed when 25.0 ml of 0.200M AgNO\(_{3}\) is mixed with 50.0 ml of 0.800 M Na\(_{2}\)CO\(_{3}\) is 0.68 g.
According the question , the chemical equation is give by :
2AgNO\(_{3}\) + Na\(_{2}\)CO\(_{3}\) -------> Ag\(_{2}\)CO\(_{3}\) + 2NaNO\(_{3}\)
Given that :
volume of silver nitrate = 25.0 ml or 0.025 L
molarity M = 0.200 M
volume of sodium carbonate = 50.0 ml or 0.050 L
molarity = 0.800 M
now, no. of moles of silver nitrate = 0.025 × 0.200 = 0.005 moles
no. of mole of sodium carbonate = 0.050 × 0.800 = 0.04 moles
the no. of moles of silver carbonate , = 0.5 × 0.005
= 0.0025 moles
molar mass of Ag\(_{2}\)CO\(_{3}\) = 275.7 g/mol
therefor, the mass of Ag\(_{2}\)CO\(_{3}\) = 0.0025 moles × 275.7 g/mol
=0.68 g
To learn more about Concentration here
https://brainly.com/question/15211488
#SPJ4
The ratio of the mass of u-238 to the mass of pb-206 can be used to.
Answers
hope this helps
What is the mass of the object?

Answers
Answer:
37.3
263.5
Explanation:
The scale measures hundreds of units, tens of units, units, and parts of units (1 decimal place.
Scale 1
Hundreds 0 * 100 = 0
Tens: 3 * 10 = 30
Units: 7 * 1 = 7
1/10 unit = 3* 0.1 = 0.3
Total 30 + 7 + 0.3 = 37.3
Scale 2
Hundreds 2 * 100 = 200
Tens: 6 * 10 = 60
Units: 3 * 1 = 3
1/10 unit = 5* 0.1 = 0.5
Total = 200 + 60 + 3 + 0.5 = 263.5