how do you think the layers of liquid would look if you put the liquids into the cylinder in a different order? why?
Answers
Answer:It actually does not matter in which order you add the three different liquids into your jar; the layers will always end up being the same: The corn syrup settles on the bottom, the colored water is in the middle and the vegetable oil floats on the top.
Explanation:
Related Questions
Which gas law can be used to calculate the pressure of hydrogen gas collected over water.
Answers
Calculating the tension of hydrogen gas gathered over water is possible using Dalton's Law of Equivalent Pressures.
Is hydrogen a liquid or a gas?Around room temperature and pressure, hydrogen is a gas, but at -423 degrees Fahrenheit, it condenses into a liquid (minus 253 degrees Celsius).
Can fuel be made from hydrogen?The gasoline for engines with internal combustion can also be hydrogen. These, however, are less effective than FCEVs and emit tailpipe pollutants. Try reading up on fuel cells. A gallon of gasoline (6.2 kilograms, 2.8 kilograms) has approximately the same amount of energy as 2.2 gbp (1 kilogram) of hydrogen.
To know more about Hydrogen visit:
https://brainly.com/question/28937951
#SPJ4
_______ is force divided by area.
Answers
Answer:
Pressure
Explanation:
Basically when you are pressing something you are applying force over an area thus Pressure is force divided by area.
Answer:
pressure
Explanation:
An asteroid in space has traveled 4,500 km in 60 s, what is the average speed of the asteroid?
Answers
When hcl is added to pure water, hcl molecules lose protons, while water molecules gain protons. in this reaction, hcl is a(n):________
Answers
When hcl is added to pure water, hcl molecules lose protons, while water molecules gain protons. in this reaction, hcl is an acid.
A substance which reacts with certain metals release hydrogen ions into water and produces salts. Acids give various dyes a reddish tints and it have a sour flavour. Some body-produced acids, a well known stomach acid, which support organ function. HCl is a specific type of acid.
Acids is divided basically into two categories:
Organic acid and inorganic acid. Another name of organic acid is mineral acids. In general, inorganic acid are strong as compared to organic acid. The major difference between these two is that organic compounds contain carbon atoms whereas inorganic compound or acid do not.
Thus, when HCl is added to pure water, HCl molecules lose protons, while water molecules gain protons. in this reaction, hcl is an acid.
learn more about HCl:
https://brainly.com/question/11800766
#SPJ4
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
21. What is the frequency, given 2-3 x 10¹m? Show all work
Answers
Answer:
Frequency is 2Hz
Explanation:
Fractional distilation of liquid air usually produces nitrogen and oxygen as major products . Substance used to remove carbon iv oxide from air before its changed to liquid
Answers
Answer:
Caustic soda
Explanation:
The fractional distillation of air is carried out on liquid air. Before air is liquified, the carbon dioxide content of air is removed using caustic soda. The air is then compressed to a pressure of about 200 atm, sudden expansion of the gas leads to cooling. The process continues until air becomes liquid at -200°C.
Fractional distillation of liquid air usually produces nitrogen and oxygen as major products. nitrogen in obtained first since it has a lower boiling point than oxygen. The gases are then dried, compressed and stored in cylinders.
given the following data C =66.7% H =11.1% Calculate the empirical formula of the compund
Answers
First, we calculate the moles of each element taking the percentages as a mass:
\(66,7g\text{ C}\cdot\frac{1\text{ mol C}}{12\text{ g C}}=5,56\text{ mol C}\)\(11,1\text{ g H}\cdot\frac{1\text{ mol H}}{1\text{ g H}}=11,1\text{ mol H}\)We divided the number of moles by the smaller number of moles. In this case, C is the smallest:
\(5,56\text{ mol C/5,56 =1}\)\(11,1\text{ mol H/5,56=1,99}\approx2\)These numbers give us the empirical formula wich is: CH2
32)
In some water purification plants, solid matter is skimmed off the top of the water. This is separating a mixture by what method?
A)
chromatography
B)
evaporation
floatation
D)
sifting
Answers
What are the names of the ions Ba^2+, Sn^2+, and Se^2-? a. barium, tin, and selenium b. barium, tin(ll), and selenide c. borium(ll), tin(ll). and selenium(ll-) d. barous. stannous, and selenide e. None of the above
Answers
The names of the ions Ba^2+, Sn^2+, and Se^2- are barium, tin(II), and selenide, respectively.
Ba^2+ is a cation (positively charged ion) formed by the loss of two electrons from the neutral atom of barium (Ba). The name of the cation is simply "barium", which is the name of the metal element.
Sn^2+ is also a cation formed by the loss of two electrons from the neutral atom of tin (Sn). In this case, the name of the cation is "tin(II)", which indicates that the ion has a +2 charge.
Se^2- is an anion (negatively charged ion) formed by the gain of two electrons by the neutral atom of selenium (Se). The name of the anion is "selenide", which is the name of the ion formed by selenium.
The correct answer is (b) barium, tin(II), and selenide
To know more about Ions ,visit:
https://brainly.com/question/30663970
#SPJ11
Gas occupies 30 Liters at 2.0 atm pressure and 27° Celsius. How many moles of gas are present
Answers
how many molecules are there in 18.5 moles of water
Answers
Answer: The answer is 18.01528.
Explanation:
why there is presence of double or triple bond between carbon?
Answers
Answer:
Alkynes
Explanation:
Alkynes are hydrocarbons which contain carbon-carbon triple bonds. Their general formula is CnH2n-2 for molecules with one triple bond (and no rings). Alkynes undergo many of the same reactions as alkenes, but can react twice because of the presence of the two p-bonds in the triple bond.
I will give you Brainly if you awsers right pl if your gonna awser for pints just put I don’t know

Answers
Mono- one
Di- two
Tri- three
Tetra- four
Penta- five
Hexa-six
Hepta- seven
Octa- eight
Nona- nine
Deca- ten
I believe the crrect answer is B) two
IamSugarBee
Luke's collection has 27 postcards in it than brian's collection. Put togteher, their collections have 135 postcards how many postcards does luke have how many postcards does brian have
Answers
Answer:
I. Luke has 81 postcards.
II. Brian has 54 postcards.
Explanation:
Let Luke's collection be L
Let Brian's collection be B
Translating the word problem into an algebraic equation, we have;
L = 27 + B
L + B = 135
Substituting equation 1 into equation 2, we have;
27 + B + B = 135
27 + 2B = 135
2B = 135 - 27
2B = 108
B = 108/2
B = 54
Therefore, Brian has 54 postcards.
To find the value of L;
L = 27 + B
L = 27 + 54
L = 81
Therefore, Luke has 81 postcards
What is the empirical formula of a compound composed of 72. 36% fe and 27. 64% o by mass?.
Answers
The empirical formula of the compound is Fe₂O₃, representing the simplest mole ratio of Fe and O.
The empirical formula represents the relative proportion of elements in a compound, showing the simplest, whole-number ratio of atoms.
It is often referred to as the molecular formula. While molecular formulas provide the actual number of atoms in a molecule, empirical formulas convey the ratios of the elements.
In the given compound composed of 72.36% Fe and 27.64% O by mass, the empirical formula is Fe₂O₃.
To determine the empirical formula:
Assume 100 g of the compound.
Calculate the number of moles for each element.
Find the ratio of moles for each element.
Divide each mole value by the smallest mole value to obtain the simplest mole ratio.
Write the empirical formula.
Following these steps:
Assuming 100 g of the compound, there are 72.36 g of Fe and 27.64 g of O.
Calculate the moles of Fe: Moles of Fe = (72.36 g Fe) / (55.85 g/mol) = 1.294 mol Fe
Calculate the moles of O: Moles of O = (27.64 g O) / (16 g/mol) = 1.7275 mol O
The ratio of moles is Fe:O = 1.294 mol Fe : 1.7275 mol O
Dividing each value by the smallest value of 1.294, we get Fe:O = 1 : 1.33
The empirical formula is Fe₂O₃.
To know more about Empirical formula here: https://brainly.com/question/1603500
#SPJ11
How do amphibians start life?
as tadpoles
as hatchlings on land
as eggs that are laid in the water
as small frogs
Answers
Answer:Most amphibians start their life cycle as eggs that are laid in the water. The eggs hatch into larvae, which are commonly referred to as tadpoles. Tadpoles spend their early life in the water, feeding on algae and other plant material. As they grow and develop, they undergo metamorphosis, which is a process where they transform into terrestrial adults. During metamorphosis, the tadpoles develop legs and lungs, and their tails are reabsorbed. Once the metamorphosis is complete, the young amphibians are able to live on land and breathe air. This life cycle is common to most amphibians, including frogs, toads, and salamanders.
Explanation:
Answer:
Amphibians typically lay eggs in water which hatch into larvae (aquatic juveniles). These larvae undergo metamorphosis and eventually emerge from the water as land-dwelling adults. Some species may also reproduce on land through a process called oviparity where they lay eggs directly onto land.
May I please have brainliest?
reduction of 1-decyne to decane requires how many equivalents of hydrogen gas?
Answers
The reduction of 1-decyne to decane requires two equivalents of hydrogen gas. This is because the reaction involves the addition of two hydrogen atoms to the triple bond of the 1-decyne molecule, resulting in the formation of a decane.
Each hydrogen atom contributes one electron to form a bond with one of the carbon atoms in the triple bond, and the reaction requires two such additions to convert the triple bond to a single bond. Therefore, two equivalents of hydrogen gas are needed to provide the necessary hydrogen atoms for the reduction to take place.
The reaction is typically carried out using a palladium or platinum catalyst under mild conditions of temperature and pressure. The reduction of alkynes to alkanes is an important organic reaction with many applications in organic synthesis and industrial processes.
You can learn more about reduction at: brainly.com/question/28813812
#SPJ11
You need to make an aqueous solution of 0.173 M zinc fluoride for an experiment in lab, using a 500 mL volumetric flask. How much solid zinc fluoride should you add
Answers
Answer:
About 8.94.
Explanation:
Because we are given a 500. mL volumetric flask, the solution will have a volume of 500. mL.
Find the number of moles of zinc fluoride needed. Recall that molarity is simply moles per liter of solution:
\(\displaystyle 500.\text{ mL} \cdot \frac{0.173\text{ mol ZnF$_2$}}{1\text{ L}} \cdot \frac{1\text{ L}}{1000\text{ mL}} = 0.0865\text{ mol ZnF$_2$}\)
Convert this to grams. The molecular weight of zinc fluoride is 103.38 g/mol:
\(\displaystyle 0.0865\text{ mol ZnF$_2$} \cdot \frac{103.38\text{ g ZnF$_2$}}{1\text{ mol ZnF$_2$}} = 8.94\text{ g ZnF$_2$}\)
In conclusion, about 8.94 grams of solid zinc fluoride should be added.
Which statement is correct about the changes in fauna’s during one of these five major events
Answers
Answer:
source Mass extinctions are episodes in which a large number of plant and animal species become extinct within a relatively short period of geologic time—from possibly a few thousand to a few million years. ... Permian Period — 252 million years ago. Devonian Period — 359 million years ago. Ordovician Period — 443 million years
BP: Nowadays, scientists are aware of five mass extinction events in the past, starting with the End-Ordovician Extinction 450 million years ago and up to the End-Cretaceous Extinction that killed off the dinosaurs 66 million years ago
In your own words- Mass extinctions area unit episodes during which an oversized variety of plant and animal species become extinct at intervals a comparatively short amount of earth science time—from probably some thousand to some million years. ... Permian period — 252 million years past. period — 359 million years past. geological period — 443 million years
BP: today, scientists area unit alert to 5 mass extinction events within the past, beginning with the End-Ordovician Extinction 450 million years past and up to the End-Cretaceous Extinction that killed off the dinosaurs sixty six million years past
Explanation:
source is where i got this information and the in your own words is it fully rewritten hope this helps and my aplogies for making this so lengthy!
Which of the following species exhibit resonance? NO3– SO3^2–; PO3^3–
A) NO3– only B) PO3^3– only C) NO3– and SO3^2–
D) SO3^2– and PO3^3–
E) NO3–, SO3^2–, and PO3^3–
Answers
The species that exhibit resonance are NO3–, SO3^2–, and PO3^3–.
Resonance refers to the delocalization of electrons within a molecule, resulting in multiple resonance structures. In NO3– (nitrate ion), the three oxygen atoms form a resonance hybrid, with the negative charge spread over the entire molecule.
Similarly, in SO3^2– (sulfite ion), the sulfur atom shares its electrons with three oxygen atoms, resulting in resonance structures. Lastly, in PO3^3– (phosphite ion), the phosphorus atom forms resonance structures by sharing electrons with three oxygen atoms.
Resonance stabilizes the molecules by distributing the charge or electron density more evenly, making them more stable and less reactive. Therefore, the correct answer is E) NO3–, SO3^2–, and PO3^3–.
To know more about resonance structures refer here:
https://brainly.com/question/25022370
#SPJ11
t/f do not use oil-based products (vaseline, body lotions) because they destroy latex
Answers
True. Do not use oil-based products such as Vaseline and body lotions because they destroy latex. Latex is a natural rubber, and when it comes into contact with oil-based products, it reacts chemically.
This reaction causes latex to degrade and lose its elasticity, making it prone to breakage. Therefore, it is important to avoid oil-based products when using latex products, such as condoms, gloves, and other medical supplies. Instead, use water-based products that are safe to use with latex. Water-based products are gentle on the skin and do not react chemically with latex, making them ideal for use with latex products.
learn more about chemically
https://brainly.com/question/12145141
#SPJ11
Simple compounds are built up and used to manufacture cellular materials in the process of ________.
Answers
Simple compounds are built up and used to manufacture cellular materials in the process of metabolism. Metabolism is the sum of all the chemical reactions that occur in the body. It's the process of breaking down large molecules into smaller ones, and building them back up into new molecules for use in cellular growth, repair, and energy production.
Metabolic processes can be divided into two categories: catabolic and anabolic. Catabolic reactions break down larger molecules into smaller ones, releasing energy in the process. Anabolic reactions build larger molecules from smaller ones, consuming energy in the process. Simple compounds like glucose, amino acids, and fatty acids are the building blocks for larger molecules like carbohydrates, proteins, and lipids. These larger molecules are essential for cellular growth, maintenance, and function. For example, proteins are made up of amino acids, which are linked together by peptide bonds to form long chains. These chains can then fold into specific three-dimensional structures, giving them unique functions in the body. Metabolism also plays a crucial role in energy production. The breakdown of glucose, for example, releases energy that can be used to power cellular processes. This energy is stored in the form of ATP (adenosine triphosphate), which can be used by the cell whenever it needs energy. Overall, the process of metabolism is essential for cellular growth, maintenance, and function. Simple compounds like glucose, amino acids, and fatty acids are used to build larger molecules like proteins, carbohydrates, and lipids, which are critical for cellular function. Additionally, metabolism plays a vital role in energy production, allowing cells to carry out the processes necessary for life.For such more question on molecules
https://brainly.com/question/24191825
#SPJ11
metals react with ? what

Answers
Answer:
metals can react with acid, water and oxygen
Complete the following radioactive decay problem. Please help

Answers
Answer:
230 90Th
Explanation:
A careful observation of the equation given in question shows that 234 92U is undergoing alpha decay. This means that the resulting daughter nuclei will have a decrease of 4 in the mass number and a decrease of 2 in the atomic number.
Please see attached photo for further details.
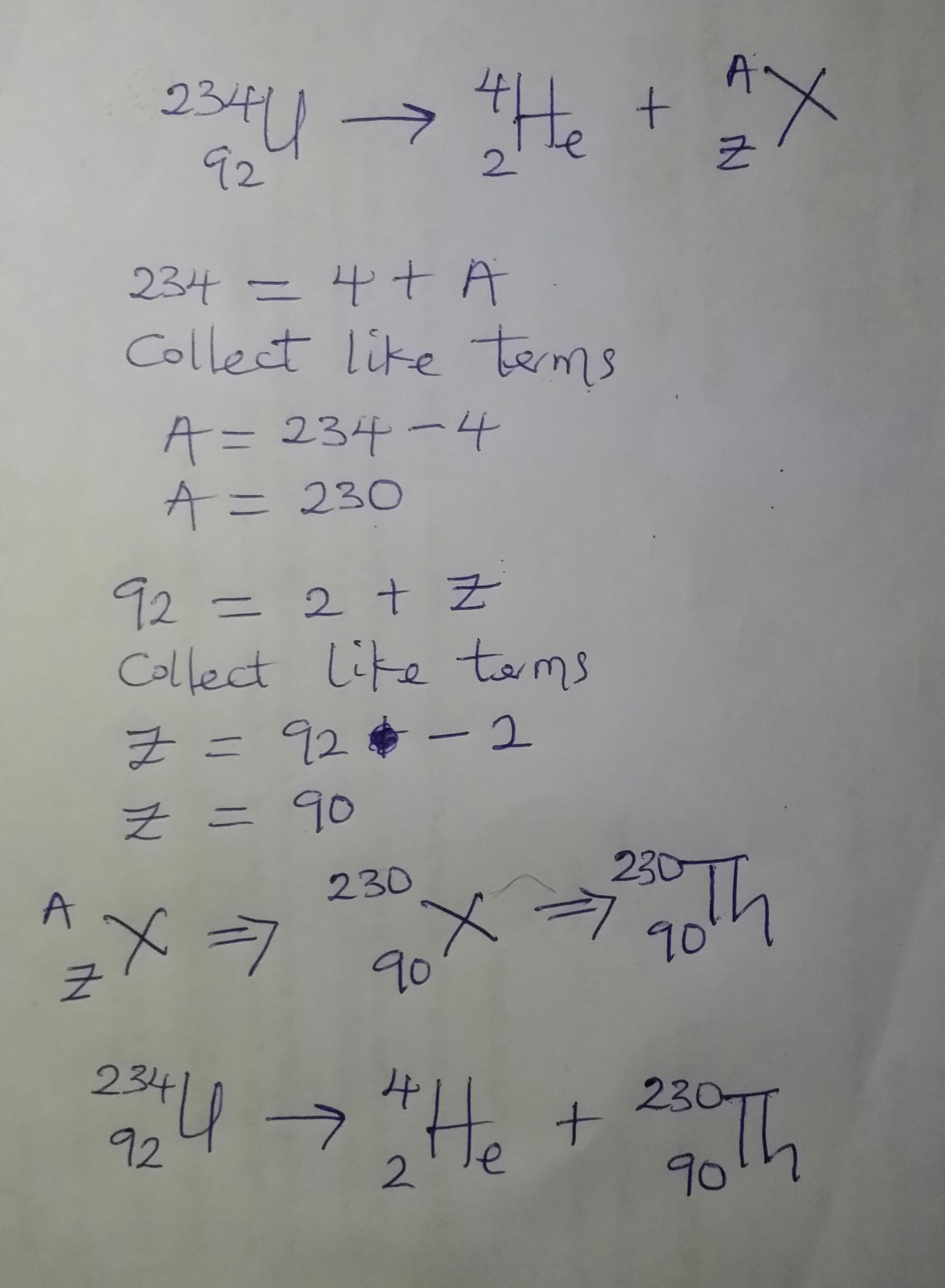
a 12.5 ml sample of 0.25 m h2so4 solution is completely neutralized by 0.150 m koh solution. calculate the volume of koh solution used in this neutralization reaction.
Answers
Volume of H2SO4 solution = 12.5 m LAs we know that, Molarity = (No. of moles of solute) / (Volume of solution in litres) Molarity of H2SO4 solution = 0.25 MNo. of moles of H2SO4 in 12.5 mL
= 0.25 × 12.5 / 1000
= 0.003125 mol As we know that.
From the balanced chemical equation,1 mole of H2SO4 reacts with 2 moles of KOH. To calculate the moles of KOH, we can use the mole ratio of H2SO4 to KOH. No. of moles of KOH = 2 × 0.003125 = 0.00625 Molarity of KOH solution = 0.150
MV = (No. of moles of solute) / (Molarity of solution).
Volume of KOH solution = No. of moles of KOH / Molarity of KOH solution= 0.00625 / 0.150= 0.0416
L= 41.6 mL Therefore, 41.6 mL of KOH solution is used in this neutralization reaction.
To know more about solution visit:
https://brainly.com/question/1616939
#SPJ11
A carboxylic acid reacts with water to form a carboxylate ion and H3O+. Complete the reaction

Answers
The carboxylate ion formed is (CH3)2CHCH2COO^- and H3O+.
What is carboxylate ion?
The carboxylate ion is the conjugate base of carboxylic acid with the group RCOO-. This can be formed by adding a base to carboxylic acid where it is deprotonated to give carboxylate anion. It contains a single negative charge because of the absence of H+.
The reaction of carboxylate ion formation can be written as,
(CH3)2CHCH2COOH + H2O -------> (CH3)2CHCH2COO- + H3O+
Here the proton of the carboxylic acid is taken by water and water acts as a base. Then the water forms hydronium ion and carboxylic acid forms carboxylate ion.
Therefore, the carboxylate ion formed by the reaction of (CH3)2CHCH2COOH with water is (CH3)2CHCH2COO- ion having IUPAC name 3-methylbutanoate.
To learn more about carboxylate ion click on the given link https://brainly.com/question/15461099
#SPJ1
A student places a 2.0 gram sample of magnesium metal in a bottle and fits the bottle with a two hole rubber stopper as shown in the diagram. Hydrochloric acid is added to the bottle and a
reaction occurs. As the reaction proceeds hydrogen gas travels through the tubing to an inverted
bottle filled with water, displacing some of the water in the bottle.
1. Calculate the number of grams of magnesium the students should mass out, to make 0.40 Liters of Hydrogrm gas at STP, show set up

Answers
The required number of grams of magnesium to make 0.40 Liters of Hydrogen gas at STP is 0.43 grams.
What is ideal gas equation?Ideal gas equation gives idea about the behavior of gas at different condition & represented as:
PV = nRT, where
P = standard pressure = 1atmV = volume = 0.4 Ln = moles = ?R = universal gas constant = 0.082 L.atm / K.molT = standard temperature = 273.15KOn putting values, we get moles as:
n = (1)(0.4) / (0.082)(273.15) = 0.0178 mol
Given chemical reaction is:
Mg + 2HCl → MgCl₂+ H₂
From the stoichiometry of the reaction:
0.0178 moles of H₂ = produced by the reaction 0.0178 moles of Mg
Mass of Mg = (0.0178mol)(24.3g/mol) = 0.43g
Hence required mass of magnesium is 0.43g.
To know more about mass & moles, visit the below link:
https://brainly.com/question/20562198
#SPJ1
onsider the reaction ch4(g) h2o(g)3h2(g) co(g) using the standard thermodynamic data in the tables linked above, calculate g for this reaction at 298.15k if the pressure of each gas is 16.00 mm hg.
Answers
To calculate the standard free energy change (ΔG°) for the reaction CH4(g) + H2O(g) → 3H2(g) + CO(g) at 298.15 K and a pressure of 16.00 mm Hg for each gas, we need to use the following equation:
ΔG° = ΣnΔGf°(products) - ΣnΔGf°(reactants)
where ΔGf° is the standard molar free energy of formation, n is the stoichiometric coefficient of the reactant or product in the balanced chemical equation.
Using the standard thermodynamic data from the tables linked above, we have:
ΔGf°(CH4,g) = -50.8 kJ/mol
ΔGf°(H2O,g) = -228.6 kJ/mol
ΔGf°(H2,g) = 0 kJ/mol
ΔGf°(CO,g) = -137.2 kJ/mol
Substituting these values into the equation above, we get:
ΔG° = (3 mol x 0 kJ/mol) + (-137.2 kJ/mol) - [(1 mol x (-50.8 kJ/mol)) + (1 mol x (-228.6 kJ/mol))]
ΔG° = -137.2 kJ/mol + 279.4 kJ/mol
ΔG° = 142.2 kJ/mol
To convert kJ/mol to J/molecule, we need to multiply by 1000 and divide by Avogadro's number:
ΔG° = (142.2 kJ/mol x 1000 J/kJ) / 6.022 x 10^23 molecules/mol
ΔG° = -2.36 x 10^-19 J/molecule
Therefore, the standard free energy change for the reaction at 298.15 K and a pressure of 16.00 mm Hg for each gas is -2.36 x 10^-19 J/molecule.
Learn more about energy change:
https://brainly.com/question/29339318
#SPJ11
2. When two oppositely charged particles are brought near each other, they produce a torceted the stone
shows the force around two changed particles
Which force do the arrows represent?
A an unbalanced force
B. a frictional force
C.an electrical force
D.a gravitational force
Answers
Answer:
A
Explanation:
An unbalanced force is your answer.