How many valence electrons are there in the P3–
ion?
A 3
B 5
C 8
D 15
E 18
Answers
P³⁻ has two electrons in its second subshell, the 2s, and six in the second subshell, the 2p. In the outermost shell, there are eight electrons overall. With 8 valence electrons as a result, the P³⁻ is.
What is electrons?An fundamental electric charge of -1 is present in the subatomic particle known as the electron. Given that they have no known components or substructure and are a member of the first generation of lepton particles, electrons are typically regarded as elementary particles. The smallest atom-forming particle and carrier of a negative charge is the electron. A neutral atom has the same number of protons and electrons. Just one electron and one proton are present in the hydrogen atom, for instance. As a result of its 92 protons and 92 electrons, the uranium atom is different.Electrons are found outside the nucleus, as opposed to protons and neutrons, which are found inside the nucleus at the heart of the atom.To learn more about electron, refer to:
https://brainly.com/question/25674345
Related Questions
A buried body of shale is subjected to differential stress, causing clay minerals to realign and produce slate. This is an example of
Answers
Metamorphism, The process of metamorphism can result in various types of metamorphic rocks depending on the original rock type and the conditions of the metamorphic process.
Metamorphism is the process of transforming one type of rock into another through heat, pressure, and/or chemical processes. In the case of the buried shale body, the differential stress applied to the rock caused the clay minerals to realign and form slate, which is a type of metamorphic rock.
In this scenario, a buried body of shale undergoes differential stress, which causes the clay minerals within it to realign and ultimately produce slate. This process is known as metamorphism, which is the alteration of a rock's mineralogy, texture, or chemical composition due to changes in temperature, pressure, or the presence of fluids within the Earth's crust.
To know more about process visit:
https://brainly.com/question/29016702
#SPJ11
Can somebody help me with these (in the pictures) I’m confused! Thanks!
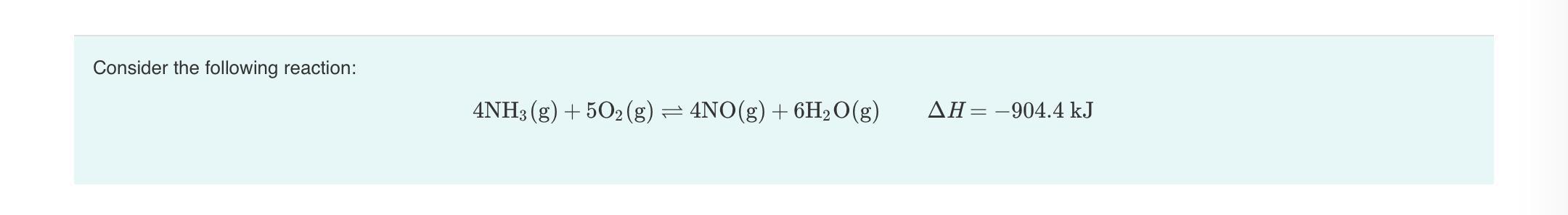

Answers
At chemical equilibrium, changing any of the parameters would not change the concentration of the product.
What is chemical equilibrium?Chemical equilibrium is defined as the condition which arises during the course of a reversible chemical reaction with no net change in amount of reactants and products.A reversible chemical reaction is the one wherein the products as soon as they are formed react together to produce back the reactants.
At equilibrium, the two opposing reactions which take place take place at equal rates and there is no net change in amount of the substances which are involved in the chemical reaction.At equilibrium, the reaction is considered to be complete . Conditions which are required for equilibrium are given by quantitative formulation.
Learn more about chemical equilibrium,here:
https://brainly.com/question/3920294
#SPJ1
Which of the following will require the least time for a reaction to reach equilibrium? O a. Cannot tell, since the time required to reach equilibrium does not depend on Kc. O b. Cannot tell without knowing the value of Kc- O c. Kc is a very large number. O d. Kc is a very small number. O e. Kc is approximately one.
Answers
The time required for a reaction to reach equilibrium can depend on the value of Kc, which represents the equilibrium constant. The equilibrium constant, Kc, is determined by the concentrations of the reactants and products at equilibrium.
In general, reactions with a larger Kc value tend to reach equilibrium more quickly than those with a smaller Kc value. This is because a larger Kc indicates that the concentration of products is higher compared to the concentration of reactants at equilibrium. As a result, the reaction proceeds more rapidly to reach the point where the ratio of products to reactants matches the value of Kc.
Therefore, among the given options, the answer would be option (c) where Kc is a very large number. In this case, the reaction would require the least amount of time to reach equilibrium.
It's important to note that the actual time required for a reaction to reach equilibrium depends on various factors such as temperature, pressure, and the presence of catalysts. Additionally, the time required for a reaction to reach equilibrium cannot be determined solely based on the value of Kc. However, in general, a larger Kc value suggests a quicker attainment of equilibrium.
Learn more about equilibrium here :-
https://brainly.com/question/14281439
#SPJ11
what is chemistry?
a. the study of changing ideas
b. the study of how matter
and energy react
c. the study of living things
d. the scientific study of matter
Answers
Answer:
B
Explanation:
Chemistry is the study of matter, its composition and the changes it undergoes.
Answer:
The scientific study of matter
Explanation:
reviewed the work, its correct
I turned the assignment in, don't mind this.
Answers
Answer: Wowwwww
Explanation:yes
Which salt is produced when hydrobromic acid (HBr) is neutralized by sodium hydroxide
(NaOH)?
A) NaBr
B) BrOH
C) NaH
D) HNa
Answers
Answer:
NaOH
Explanation:
the balance equation is HBr +NaOH----> NaBr+ H2O. The salt formed is NaBr, sodium,Bromide
How do you decide between SN1 and SN2?
Answers
The two types of nucleophilic substitution reactions are SN1 and SN2. SN2 has two molecules, whereas SN1 only has one.
What is Nucleophilic substitution reaction?A nucleophilic molecule replaces a different atom or group of atoms on a molecule, known as the leaving group, in a nucleophilic substitution reaction. The substrate molecule is attacked by the nucleophilic molecule's abundant electrons.
A process in which one functional group or atom is swapped out for another negatively charged functional group or atom is known as a nucleophilic substitution reaction.
The SN1 reaction is monomolecular, whereas the SN2 reaction is bimolecular.
Any substitution reaction in which an atom or functional group is changed for one that has a single pair of electrons, a negatively charged ion, or both. The negatively charged ion or the atoms/molecules with lone pairs of electrons will be pulled to the positively charged area of an atom or complex in an effort to replace the functional group or atom already attached to the positive location.
Therefore, The two types of nucleophilic substitution reactions are SN1 and SN2. SN2 has two molecules, whereas SN1 only has one.
To know more about Nucleophilic substitution, refer to the link:
https://brainly.com/question/32657850
#SPJ6
You have 2.7 moles of carbon. How many atoms do you have?
Answers
Answer:
1.63 × 10²⁴ atoms.
Explanation:
To calculate the number of atoms (N) contained in 2.7moles of carbon, we multiply the number of moles (n) by Avogadro's number (6.02 × 10²³).
That is, N = n × nA
Where;
N = number of atoms
n = number of moles (mol)
nA = Avogadro's numbe
N = 2.7 × 6.02 × 10²³
N = 16.254 × 10²³
N = 1.63 × 10²⁴ atoms.
Hence, there are 1.63 × 10²⁴ atoms in 2.7moles of Carbon.
Please give detailed solution with CLEAR EXPLANATION AND ALL THE
REASONS. Thank you.
Wascana Chemicals produces paint and emits sulphur dioxide during production. However, the Ministry of Environment mandates all paint firms to reduce emissions. Answer the questions below using the gi
Answers
Wascana Chemicals should use emissions reduction technologies to reduce the amount of sulphur dioxide emitted during paint production.
To comply with the Ministry of Environment's directive, Wascana Chemicals, a paint manufacturer, needs to reduce the amount of sulphur dioxide released during paint production. This can be accomplished through the use of emissions reduction technology, such as scrubbers, catalytic converters, or gasification systems.Scrubbers are devices that use a wet process to remove pollutants from gas streams. The gas stream is forced through a scrubbing solution that traps pollutants, including sulphur dioxide.Catalytic converters, on the other hand, use a chemical process to transform pollutants into less harmful substances. Gasification systems convert solid or liquid materials into a gas, which can be combusted to generate energy.
In conclusion, to comply with the Ministry of Environment's emissions reduction regulations, Wascana Chemicals should consider implementing one or more emissions reduction technologies such as scrubbers, catalytic converters, or gasification systems to reduce the amount of sulphur dioxide emitted during paint production.
To know more about Scrubbers visit:
brainly.com/question/30042938
#SPJ11
Calculate the oxidation number of sulphur in H2SO4
Answers
Answer:
42
Explanation:
my brother said
Part A: Calculate the percent ionization of 0.135 M lactic acid (Ka=1.4×10−4). Express the percent ionization to two significant digits.
Part B: Calculate the percent ionization of 0.135 M lactic acid in a solution containing 6.5×10^−3 M sodium lactate. Express the percent ionization to two significant digits.
Answers
1) The percentage ionization is 3.2%
2) The percentage ionization is 21.9%
What is the percent ionization?The percent ionization is primarily used for weak acids and bases, where only a fraction of the compound dissociates. Strong acids and bases, on the other hand, completely dissociate in solution, so the percent ionization is 100%.
We have to use the formula;
α = √ka/C * 100
Where;
α = percent ionization
Ka = The dissociation constant
C = concentration
Then;
α = √1.4×10^−4/0.135 * 100
= 3.2 %
2) α = √6.5×10^−3/0.135 * 100
= 21.9%
Learn more about percent ionization:https://brainly.com/question/1619653
#SPJ4
TEXT ANSWER
How and why have scientists developed various models of the atom?
Practice
My scientific explanation is:
Your answer should include at least six complete sentences to explain the claim, evidence and reasoning.
Be sure to check your grammar and spelling.
Answers
Answer:Scientists used the model to make predictions. Sometimes the results of their experiments were a surprise and they did not fit with the existing model. Scientists changed the model so that it could explain the new evidence. The discovery of electrons. Atoms can be broken down into smaller parts.
Explanation:
The bond that forms from the
side-to-side overlap of orbitals of
two atoms is termed a _
bond.
А
sigma
B
pi
Answers
Answer:
pi
Explanation:
In chemistry, a covalent bond is formed by the overlap of atomic orbitals. The bond formed may be a sigma bond or a pi bond depending on the way the atomic orbitals are overlapped.
If the overlap occurs in a side-to-side manner, the bond formed is said to be a pi bond. Pi bonds are common in organic species having multiple bonds such as ethene and benzene.
what particle determines the name of the element?
Answers
Answer:
The protons determine the name of an element and also atomic number because its the same as protons.
Where is most of the high-level waste stored in the United States?
salt mines
at the nuclear reactor site
mountain peaks
the ocean
volcanic fissures
Answers
Answer:
at the nuclear reactor site
Explanation:
In the United States, liquid high-level waste is stored in underground tanks pending vitrification. High-level waste is stored at the nuclear reactor site.
Such waste was created by the weapons programs of the cold war and the Manhattan project. This waste can not be vitrified as there were not enough funds for further processing.
Answer:
at the nuclear reactor site
Explanation:
A lump of zinc is tossed into a beaker of 500L of 14M hydrochloric acid. this reaction produces Hydrogen Gas and zinc (II) chloride. If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, what is the mass of the zinc?
Answers
If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, 2796.96 g mass of the zinc is produced .
Using the ideal gas law equation:
PV = nRT
n = (PV) / (RT)
= (1.75 atm * 645 L) / (0.0821 atm·L/(mol·K) * 400 K)
= 42.71 moles
the balanced equation for the reaction between zinc and hydrochloric acid:
Zn + 2HCl -> \(ZnCl_{2}\) + \(H_{2}\)
1 mole of zinc produces 1 mole of hydrogen gas. Therefore, the moles of zinc are also 42.71.
The molar mass of zinc is 65.38 g/mol.
Mass of zinc = moles of zinc * molar mass of zinc
= 42.71 moles * 65.38 g/mol
= 2796.96 g
Therefore, the mass of the zinc is 2796.96 grams.
learn more about hydrogen gas :
https://brainly.com/question/30829657
HURRYYY!!!!
How many moles of salt do you have, if you have 5.00 x 10^26 formula units of salt?
Answers
Answer:
830.56 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L}\\\)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
\(n = \frac{5.00 \times {10}^{26} }{6.02 \times {10}^{23} } \\ = 830.5647..\)
We have the final answer as
830.56 molesHope this helps you
what happens when ethylene gas is passed through cold dilute alkaline kMnO4 solution
Answers
Answer:
it gives CH2OH-CH2OH(ethylene glycol +MnO2
Explanation:
if you had a buffer (buffer c) in which you mixed 8.203 g of sodium acetate
Answers
If you mixed 8.203 g of sodium acetate in a buffer solution, we can calculate the concentration of sodium acetate in the solution.
First, we need to determine the number of moles of sodium acetate using its molar mass. The molar mass of sodium acetate (CH3COONa) is approximately 82.03 g/mol.Number of moles of sodium acetate = mass / molar mass
Number of moles of sodium acetate = 8.203 g / 82.03 g/mol
Number of moles of sodium acetate ≈ 0.1 mol Next, we need to consider the volume of the solution in which the sodium acetate is dissolved. Without this information, we cannot determine the concentration of sodium acetate accurately.If you provide the volume of the solution, we can calculate the concentration by dividing the number.
To know more about number visit :
https://brainly.com/question/3589540
#SPJ11
An ecosystem is all the populations of organisms that live together and
A.
the food that they take in as energy.
B.
the organisms that generate energy from sunlight.
C.
the microorganisms that depend on them.
D.
the physical factors with which they interact.
Plllzzzzz help :(
Answers
Answer:
D i belive
Explanation:
what is one of the causes of mechanical weathering
Answers
Answer:
Mechanical weathering is usually caused by extreme hot and cold temperatures. Water seeps into cracks in rocks, freezes, and expands, causing further breakdown of rocks. Wind is another example of mechanical weathering.
Explanation:
Answer:
carbon dioxide i think
Explanation:
Would you expect the heterogeneous method to give a higher percent nickel or a lower percent nickel than the homogenous method? Explain your answer.
(From the Quantitative Determination of Nickel in a Compound By Gravimetric Analysis lab)
Answers
Answer:
Heterogeneous method
Explanation:
In general, a gravimetric method of analysis consists
of the following steps:
Weighing and dissolving the sample in the proper solvent.
Carrying out a sequence chemical reactions to obtain a suitable product.
Determining the mass of the product
Calculating the composition of the sample rom mass of product and information about the original sample.
Precipitates obtained by heterogeneous methods are often difficult to filter and are always contaminated by adsorbed and occluded impurities which ultimately increase the percentage yield of nickel due to occluded impurities.
Which ion has the electron configuration: 1s22s22p63p64s23d104p65s24d105p6
Answers
Answer:
It would be the chloride ion, with a charge of
1
−
.
Explanation:
A neutral chlorine atom has the electron configuration
1s
2
2s
2
2p
6
3s
2
3p
5
. In an ionic compound, chlorine gains the sixth
3p
electron, most likely when reacting with a metal such as sodium (Na), forming the chloride ion,
Cl
−
.
The image below represents the formation of the ionic compound sodium chloride,
NaCl
. The bond is formed by the electrostatic attraction between the positive and negative ions.
A gas at 750 mmhg and with a volume of 2. 00 l is allowed to change its volume at constant temperature until the pressure is 600 mmhg. What is the new volume of the gas?
Answers
Answer:
The new volume of a gas at 750 mmhg and with a volume of 2. 00 l when allowed to change its volume at constant temperature until the pressure is 600 mmhg is 2.5 Liters.
Explanation:
Boyle's law states that the pressure of a given amount of gas is inversely proportional to it's volume at constant temperature. It is written as;
P ∝ V
P V = K
P1 V1 = P2 V2
Parameters :
P1 = Initial pressure of the gas = 750 mmHg
V1 = Initial pressure of the gas = 2. 00 Liters
P2 = Final pressure of the gas = 600 mmHg
V2 = Fimal volume of the gas = ? Liters
Calculations :
V2 = P1 V1 ÷ P2
V2= 750 × 2. 00 ÷ 600
V2 = 1500 ÷ 600
V2 = 2.5 Liters.
Therefore, the new volume of the gas is 2. 5 Liters.
Eva buys a package of food, and the nutrition label says that
one serving is equal to 36 grams and has 110 Calories. However, the
box is printed with a label that says 0.150 pounds. If Eva eats the
whole package, how many Calories will she have consumed?
Answers

The nutrition label is the representation of the calories provided by the molecules. If Eva eats the whole package weighing 0.150 pounds then she consumed 207.9 calories.
What is a nutrition label?The nutrition label has been seen on packaged food items and is a representation of the nutrient values given by the ingredients like the fat, carbohydrates, proteins, fats, fiber, minerals, vitamins, etc. in per serving amount.
Given,
One serving weighs = 36 grams
Calories per serving = 110 calories
Total box weighs = 0.150 pounds
It is known that 1 pound = 453.592 grams
Then, 0.150 pounds = 68.03886 grams
If 36 grams = 110 calories
Then, 68.04 grams = X calories
Solving for X as:
X = (110 calories × 68.04 grams) ÷ 36 grams
X = 207.9 calories
Therefore, Eva consumes 207.9 calories.
Learn more about nutrition labels, here:
https://brainly.com/question/13337633
#SPJ6
The pressure of x mL of a gas was increased from 2 ATM to 3 ATM at constant temperature what will be the new volume?
Answers
Answer:
2/3 xX ml of gas
Explanation:
P1V1= P2V2
2 x X= 3 x y
y (new volume)= 2/3 x
0.66 x will be the new volume when the pressure of x mL of gas was increased from 2 ATM to 3 ATM at a constant temperature.
What is an ideal gas equation?The ideal gas law (PV = nRT) relates to the macroscopic properties of ideal gases. An ideal gas is a gas in which the particles (a) do not attract or repel one another and (b) take up no space (have no volume).
Given:
\(P_1\)= 2 ATM
\(V_1\)= x mL
\(P_2\) =3 ATM
\(V_2\) =?
\(P_1V_1= P_2V_2\)
2 x X= 3 x Y
Y(new volume)= 0.66 x
Hence, 0.66 x will be the new volume when the pressure of x mL of gas was increased from 2 ATM to 3 ATM at a constant temperature.
Learn more about the ideal gas here:
https://brainly.com/question/27691721
#SPJ5
a sample of weighing 46.3 ng has decayed for 18.03 days. given that the half-life of this nuclide is 6.01 days, what mass of the original sample remains? question 24 options: 40.3 ng 5.79 ng 0 ng 223.15 ng
Answers
It is possible to determine how much of a nuclide is still present after a specific amount of time by using the equation:N0 * e(-kt) = N(twhere N(t) denotes the quantity
nuclide still present at time t, N0 denotes the nuclide's starting concentration, k denotes the decay constant, and e denotes the base of natural logarithmsThe equation below can be used to link the decay constant to the nuclide's half-life (t1/2):k = ln(2) / t1/2With the supplied values entered into these equations, we obtain:0.115 day-1 is equal to k = ln(2) / t1/2 = ln(2) / 6.01 days.The formula for N(t) is N0 * e(-kt) = N0 * e(-0.115 * 18.03 days) = 0.407 * N0.In light of this, the mass of the initial sample that is still present after 18.03 days is:m = 0.407 * 46.3 ng = 18.84where N(t) denotes the quantity of a nuclide still present at time t, N0 denotes the nuclide's starting concentration, k denotes the decay constant, and e quantity the base of natural logarithms.The equation below can be used to link the decay constant to the nuclide's half-life (t1/2):k = ln(2) / t1/2With the supplied values entered into these equations, we obtain:0.115 day-1 is equal to k = ln(2) / t1/2 = ln(2) / 6.01 days.The formula for N(t) is N0 * e(-kt) = N0 * e(-0.115 * 18.03 days) = 0.407 * N0.In light of this, the mass of the initial sample that is still present after 18.03 days is:m = 0.407 * 46.3 ng = 18.84 ngTherefore, the answer is almost 18.8 ng. As a result, the closest choice is 40.3 ng, which is incorrect.
learn more about quantity here :
https://brainly.com/question/14581760
#SPJ11
explain why it is appropriate to group a polyatomic ion in parentheses in a chemical formula, if more than one of that ion is present in the formula
Answers
Answer:
See explanation
Explanation:
It is commonplace to put polyatomic ions in parenthesis when they are more than one in a compound. A typical example here is calcium phosphate- Ca3(PO4)2.
When a compound contains more than a single polyatomic ion of the same type,a parentheses is placed around the formula of that ion then a subscript is used to indicate how many of that particular ion are in the compound.
When two ionic compounds are dissolved in water, a double replacement reaction can... Group of answer choices occur if two of the ions form an insoluble ionic compound, which precipitates out of solution occur if the ions react to form a gas, which bubbles out of the solution never occur since all ions are in water occur if the ions react to form a gas, which bubbles out of the solution ionic compounds only react with nonmetals
Answers
Answer:
occur if two of the ions form an insoluble ionic compound, which precipitates out of solution
Explanation:
When two ionic compounds are dissolved in water, a double replacement reaction takes place if two of the ions form an insoluble ionic compound, which precipitates out of solution. In double displacement reaction ions switch partners. And hence, produce an insoluble precipitate.
if the element A have three protons in the nucleus. find the atomic number of the element A
Answers
Answer:
ATOMIC number of element A is 3
Explanation:
Atomic number is the same as number of protons of an element.
according to the periodic table, Lithium is the element with 3 protons in its neutral state and it's atomic number is 3.
