not sure how to do this
the topic is limited reactants
i could do the first 2 but i’m stuck on this one because i don’t know the formula
Answers
Answer:
24 g of oxygen
Explanation:
The equations means that four moles of CH3NO2 reacts with three moles of oxygen to yield for moles of carbon dioxide, two moles of nitrogen gas and six moles of water.
The balanced equation of the reaction is;
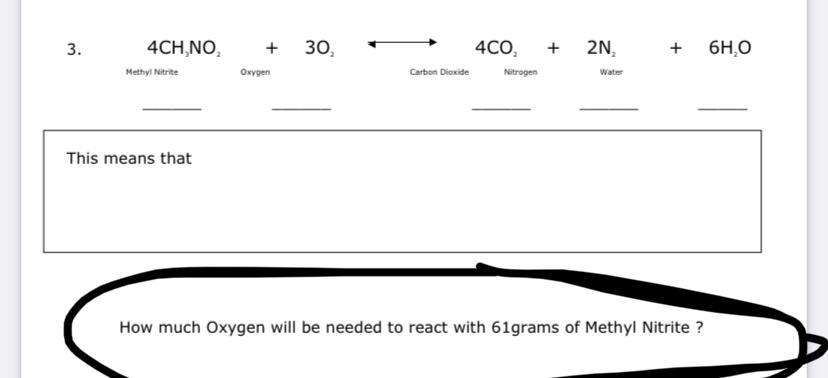
4CH3NO2 + 3O2 -----> 4CO2 + 2N2 + 6H2O
Molar mass of CH3NO2 = 61 g/mol
61 g of methyl nitrate contains 61 g/ 61 g/mol = 1 mole of CH3NO2
From the balanced reaction equation;
4 moles of CH3NO2 reacts with 3 moles of oxygen
1 mole of moles of CH3NO2 reacts with 1 * 3/4 = 0.75 moles of oxygen gas
0.75 moles of oxygen gas * 32 g/mol = 24 g of oxygen
Related Questions
Le Chatelier's Principle governs what property?A. Reaction rateB. None of theseC. EquilibriumD. Catalysts
Answers
Equilibrium. Option C is correct
Explanations:What is Le Chatelier's principle?This law states that a new equilibrium state is achieved if the changes in temperature, pressure, concentration and volume will cause a predictable and opposing changes in the system.
This shows that Le Chatelier's principle can be used to predict the properties above to determine the effect equilibrium have on a system.
Based on the above explanations, we can conclude that Le Chatelier's Principle governs the property of Equilibrium.
which element its oxide dissolves in both acids and alkalis
Answers
Answer:
Aluminum and Zinc
Explanation:
Amphoteric oxides have both acidic and basic properties. The oxides of aluminium and zinc are examples. They form salts when they react with acids. They also react with alkalis to form complex salts.
can someone please help me hurry

Answers
Answer:
The answer to the question is 148.3 g
Explanation:
To find moles, you want to get rid of the grams by finding molar mass. Then, you have to get rid of grams using cross multiplication. This is why 148.3 grams is on the bottom; it will get rid of the grams on the original amount leaving you moles.
what does Le châteliers principle state?
Answers
Hope this helps!
A solution is made by dissolving 22 grams of sodium hydroxide in water. The sodium
hydroxide solution is then titrated against an unknown solution of oxalic acid. If it takes
14.9 mL of the acid to reach the end point, what is the concentration of the oxalic acid?
NaOH + H₂C₂O → H₂O + Na₂C₂O₁
Answers
To find the concentration of the unknown oxalic acid solution, we need to use the balanced chemical equation for the reaction:NaOH + H₂C₂O → H₂O + Na₂C₂O₁
From the equation, we can see that the mole ratio between sodium hydroxide (NaOH) and oxalic acid (H₂C₂O) is 1:1. First, we need to determine the number of moles of NaOH used in the titration. The molar mass of NaOH is 22.99 + 16.00 + 1.01 = 40.00 g/mol. Therefore, the number of moles of NaOH is:moles of NaOH = mass of NaOH / molar mass of NaOH
= 22 g / 40 g/mol
= 0.55 mol
Since the mole ratio between NaOH and H₂C₂O is 1:1, the number of moles of H₂C₂O is also 0.55 mol.Now, we can determine the concentration of the oxalic acid solution using the volume of the acid used in the titration. The volume is given as 14.9 mL, which is equivalent to 0.0149 L. concentration of oxalic acid (C) = moles of H₂C₂O / volume of H₂C₂O
= 0.55 mol / 0.0149 L
≈ 36.91 mol/L.Therefore, the concentration of the unknown oxalic acid solution is approximately 36.91 mol/L.
For more such questions on oxalic acid
https://brainly.com/question/27550255
#SPJ11
Sodium hydroxide (naoh) is a strong base that is very corrosive. what is the mass of 2.75 × 10-4 moles of naoh? a. 3.24 x 10""3 g naoh b. 1.10 x 10""2 g naohc. 6.10 x 10""2 g naohd. 6.50 x 10""2 g naoh
Answers
Option B is supported by the mass of 2.75 104 moles of NaOH, which is 1.1 102 g.
To find the mass of \(2.75 x 10^-^4\) moles of NaOH, we need to use the formula:
mass = moles x molar mass
The molar mass of \(NaOH\) is 40.00 g/mol (22.99 g/mol for Na + 16.00 g/mol for O + 1.01 g/mol for H).
So, the mass of \(NaOH\) is:
mass =\(2.75 x 10^-^4\) mol x 40.00 g/mol
mass = 0.011 g
Converting this to scientific notation, we get:
mass \(= 1.1 x 10^-^2 g\)
Therefore, the answer is option b)\(1.10 x 10^-^2 g NaOH.\)
To find the mass of \(NaOH\), we need to use the formula:
mass = moles × molar mass
The molar mass of \(NaOH\) (sodium hydroxide) is approximately 40 g/mol (23 g/mol for Na, 16 g/mol for O, and 1 g/mol for H).
Given, moles of \(NaOH\) = 2.75 × 10⁻⁴
Now, let's calculate the mass:
mass = (2.75 × 10⁻⁴ moles) × (40 g/mol) = 1.1 × 10⁻² g
So, the mass of 2.75 × 10⁻⁴ moles of \(NaOH\) is 1.1 × 10⁻² g, which corresponds to option B.
For more question on mass
https://brainly.com/question/24191825
#SPJ11
Combustibilty defined?
Answers
how much energy is required to ionize hygrogen in each of the following states? (a) ground state
Answers
The energy needed is the energy that changes. The electron in a hydrogen atom is initially assumed to be in the ground state with n=1. The energy of the electron in its ground state is therefore 13.6 eV.
As a result, 12.75eV of the energy is needed to transfer electrons from the ground state to the third excited state. The 4th and 5th ionisation the energies differ significantly from one another. The fourth electron is attracted to the nucleus atom considerably less strongly than the fifth electron because it is in an inner main shell that is closer to the nucleus.
To learn more about electron, click here.
https://brainly.com/question/1255220
#SPJ4
what volume of h2 (g) (at 750. mmhg and 25 ∘ c) is produced from 50.0 ml of 0.214 m h2so4 and 0.300 g of al? 2al (s) 3h2so4 (aq) → al2(so4)3 (aq) 3 h2 (g)
Answers
The volume of hydrogen gas produced is 0.266 L, from 50.0 mL of 0.214 M H₂SO₄ and 0.300 g of Al.
To solve this problem, we need to use stoichiometry to determine how much H₂ gas will be produced from the given amounts of H₂SO₄ and Al, and then use the ideal gas law to calculate the volume of H₂ gas at the given conditions.
Balanced chemical equation for the reaction;
2Al (s) + 3H₂SO₄ (aq) → Al₂(SO₄)₃ (aq) + 3H₂ (g)
From the equation, we can see that 3 moles of H₂ are produced for every 2 moles of Al. We can use this ratio to calculate the number of moles of H₂ produced from the given mass of Al
0.300 g Al × (1 mol Al / 26.98 g) × (3 mol H₂ / 2 mol Al) = 0.0329 mol H₂
Next, let's use the volume and concentration of H₂SO₄ to calculate the number of moles of H₂SO₄ used in the reaction;
50.0 ml H₂SO₄ × (1 L / 1000 ml) × (0.214 mol / L) = 0.0107 mol H₂SO₄
Since the reaction requires 3 moles of H₂SO₄ for every 2 moles of Al, we can see that the amount of H₂SO₄ is limiting the amount of H₂ that can be produced. Therefore, we can conclude that 0.0107 mol of H₂ will be produced.
Now, we can use the ideal gas law to calculate the volume of H₂ gas produced at the given conditions;
PV = nRT
P = 750. mmHg = 0.987 atm (converting to atm)
V = unknown
n = 0.0107 mol
R = 0.0821 L atm / (mol K)
T = 25 + 273 = 298 K
Solving for V, we get;
V = nRT / P = (0.0107 mol)(0.0821 L atm / (mol K))(298 K) / 0.987 atm
= 0.266 L
Therefore, the volume of H₂ gas produced is 0.266 L.
To know more about hydrogen gas here
https://brainly.com/question/14355155
#SPJ4
What is an interference in science

Answers
A conclusion derived from evidence and logical reasoning
What is the mass of 2.3 moles of SnF4?
Answers
447.81830943999995. that's the mass
Oxalic acid (98%) is a polyprotic acid. It has a density of 1.65 g/cm^3 and a melting point of 189.5°C. Oxalic acid has a molecular mass of 90.03 g/mol and with a pka1 of 5.62 x10^-2. What volume of oxalic acid must be added to sufficient water to give a 1.500 liter solution that is 0.300 F (in formal concentration)?
Answers
Approximately 24.55 cm^3 of oxalic acid must be added to sufficient water to give a 1.500 liter solution with a formal concentration of 0.300 F.
To find the volume of oxalic acid needed to make a 1.500 liter solution with a formal concentration of 0.300 F, we need to use the equation:
Formal concentration (F) = (moles of solute) / (volume of solution in liters)
First, we need to calculate the moles of oxalic acid required. The formal concentration (F) is given as 0.300, so:
0.300 = (moles of oxalic acid) / 1.500
Rearranging the equation, we find:
moles of oxalic acid = 0.300 * 1.500
moles of oxalic acid = 0.450
Next, we can calculate the mass of oxalic acid needed using its molecular mass:
mass of oxalic acid = moles of oxalic acid * molecular mass
mass of oxalic acid = 0.450 * 90.03
mass of oxalic acid = 40.5145 g
Finally, we can calculate the volume of oxalic acid needed using its density:
volume of oxalic acid = mass of oxalic acid / density
volume of oxalic acid = 40.5145 g / 1.65 g/cm^3
volume of oxalic acid = 24.55 cm^3
Learn more about oxalic acid here :-
https://brainly.com/question/32770055
#SPJ11
How many H+(aq) ions are present in 65. 5 mL of 0. 722 M sulfuric acid?
Answers
To determine the number of H+(aq) ions present in the given solution of sulfuric acid (H₂SO₄), we need to use the molarity (M) and volume (V) of the solution.
The given information is:
Molarity (M) = 0.722 M
Volume (V) = 65.5 mL = 65.5 cm³
To find the number of H+(aq) ions, we need to consider the dissociation of sulfuric acid in water:
H₂SO₄ → 2H⁺(aq) + SO₄²⁻(aq)
From the balanced equation, we can see that for every 1 mole of sulfuric acid (H₂SO₄), it dissociates into 2 moles of H⁺(aq) ions.
First, let's convert the volume from milliliters (mL) to liters (L):
V(L) = V(mL) / 1000
V(L) = 65.5 mL / 1000 = 0.0655 L
Next, we can calculate the number of moles of sulfuric acid (H₂SO₄) in the solution using the formula:
moles = Molarity * Volume
moles(H₂SO₄) = 0.722 M * 0.0655 L
Now, we know that for every 1 mole of sulfuric acid (H₂SO₄), it dissociates into 2 moles of H⁺(aq) ions. Therefore, the number of H⁺(aq) ions can be calculated as:
moles(H⁺) = 2 * moles(H₂SO₄)
Finally, we can convert the moles of H⁺(aq) ions into the number of ions using Avogadro's number (6.022 × 10²³ ions/mol).
Number of H⁺(aq) ions = moles(H⁺) * Avogadro's number
By performing these calculations, we can determine the number of H⁺(aq) ions present in 65.5 mL of 0.722 M sulfuric acid.
Number of H⁺(aq) ions = 0.094722 moles * 6.022 × 10²³ ions/mol
Number of H⁺(aq) ions ≈ 5.701 × 10²² ions
Therefore, approximately 5.701 × 10²² H⁺(aq) ions are present in 65.5 mL of 0.722 M sulfuric acid.
Learn more about Sulfuric acid from the link given below.
https://brainly.com/question/12986533
#SPJ4
MgCl2 is electrolyzed to produce Mg and Cl2. 1311 g of MgCl2 decomposed, what is the percent yield for this reaction if 246.43 g of magnesium are recovered ?
PLEASE HELP
***write a balanced equation
Answers
The percent yield of MgCl2 is calculated as follows: (35.8 g/45.56 g) * 100% = 78.58% per mass. The actual yield od MgCl2 is 35.8 g.
MgCl2 is it a salt?The chemical formula of magnesium chloride, or MgCl2, is one magnesium & two chloride ions. It is a salt (compound). MgCl2(H2O)x is the formula for its different hydrates. As an alloying agent and for catharsis.
MgCl2 - Is it a gas?MgCl2, a solid white substance. The direct mixing of dry chlorine and magnesium results in the unknown hydrate (hexagon shaped; r.d. 2.32; p.p.m. 714°C; b.p. 1412°C): Mg(s)+Cl2(g) = MgCl2 (s) As a component of carnallite (KCl), the substance also naturally exists.
To know more about MgCl2 visit:
https://brainly.com/question/30262579
#SPJ1
Chemistry give the number of significant figures: 6.32 g
Answers
There are 3 important numbers.All digits in significant figures that are not zeros.
How can you locate important numbers in chemistry? There are three important numbers.All digits in significant figures that are not zeros.as well as all zeros that, if deleted, would NOT alter the number.I realize that the words are backwards, but they are important for indicating where the measurement finishes. Basic Guidelines for Calculating the Number of Significant Figures All digits that are not zero have meaning.With two exceptions, zeros are also significant: those that come before the decimal point.In numbers greater than one, terminal zeros before the decimal point present an uncertain situation.(1) All significant numerals are non-zero:There are 2 major figures in 1.2 g and 4 significant figures in 1.234 g.(2) Significant zeros occur between non-zero digits:3.07 mL has three significant digits and 1002 kg has four.5 - The number 48.050 has 5 major digits.
To learn more about significant figures refer
https://brainly.com/question/24630099
#SPJ1
Sabiendo el número atómico del titanio y además que su número másico es 48, ¿cuál es la cantidad de protones, neutrones y electrones respectivamente? *
Answers
Answer:
# protones: 22
# neutrones: 26
# electrones: 22
Explanation:
El número atómico del titanio es 22. Este número atómico representa la cantidad de protones del átomo en cuestión. Así:
# protones: 22.
El número másico se define como la suma de protones y neutrones, así:
48 = 22 + # neutrones
26 = # neutrones
Un átomo es neutro, esto quiere decir que sus cargas positivas y negativas son iguales (Protones: cargas positivas; Electrones: Cargas negativas).
Así, el # protones = # electrones = 22
What is the definition of gravitational potential energy?
The energy an object has because of its particle motion.
B The energy an object has because of the amount of its movements.
The energy an object has because of its position, rather than its motion.
D The energy an object could have if it applied itself.
Answers
the energy an object has because of its position rather than its motion.
The atomic number of an element indicates the number of?
Answers
Answer:
The number is mostly related to the mass or the weight of the element.
Explanation:
what is this number called?

Answers
Answer:
Atomic mass i believe..
1) A solution contains either Cr3+ or Ni2+ ion. Describe a reaction with a single reagent that will identify which ion is present.
Answers
To identify whether the solution contains Cr3+ or Ni2+ ions, a single reagent can be used. A solution of 1,10-phenanthroline in ethanol can be added to the unknown solution.
To identify whether the solution contains Cr3+ or Ni2+ ions, a single reagent can be used. A solution of 1,10-phenanthroline in ethanol can be added to the unknown solution. If Cr3+ is present, a red complex ion will form, [Cr(phen)3]3+, due to the reaction between Cr3+ and the 1,10-phenanthroline. On the other hand, if Ni2+ is present, a green complex ion will form, [Ni(phen)3]2+, due to the reaction between Ni2+ and the 1,10-phenanthroline. The color change of the solution will indicate the presence of either Cr3+ or Ni2+ ion. This reaction is known as a complexation reaction, where a ligand (1,10-phenanthroline) binds to a metal ion (Cr3+ or Ni2+) to form a complex ion with a characteristic color.
To learn more about Ethanol click here
https://brainly.com/question/25002448
#SPJ11
Which scientist developed the planetary model that stated electrons were found in specific energy levels around the nucleus?
a. Earnest Rutherford
b. Max Planck
c. Neils Bohr
d. James Cahdwick
e. J.J. Thompson
Answers
Answer: The answer is Ernest Rutherford.
Explanation:
This is atom economy and I need help ASAP. (It’s 2.2 by the way).

Answers
The atom economy of method 1 is 17%
Titanium is a valuable and expensive metal with some unique properties that make it suitable for special purposes.
What is atom economy?Titanium is the perfect material for marine and aerospace applications because it has high corrosion resistance, especially in saltwater settings. Additionally biocompatible, titanium does not react with living tissue.
We know that the formula for atom economy is;
Atom economy(%) = Mass of desired product/Mass of reactants * 100/1
Mass of desired product = 48 g
Mass of reactants = 80 + 142 + 12 + 48 = 282 g
Atom economy (%) = 48/282 * 100/1
= 17%
Learn more about atom economy:https://brainly.com/question/31978848
#SPJ1
Explain in a short sentence how you can tell a reaction is a decomposition reaction.
Answers
Answer: A decomposition reaction occurs when one reactant breaks down into two or more products.
Mark me as brainilist pls
PLEASE HELP
This tree species was preserved as a fossil in the Arizona desert. The species is now extinct. Describe what the environment was like when the tree lived and how it is different now. Then, infer why this type of tree and other living things that lived with it are extinct
Answers
When the tree species was preserved as a fossil in the Arizona desert, the environment was likely different compared to the present. Based on the fact that the tree species is now extinct, infer that the conditions that supported its existence have significantly changed.
What leads to extinction?In the past, the environment where the tree lived may have had different climatic conditions, such as temperature, precipitation patterns, and humidity levels. It may have been part of a specific ecosystem or habitat that provided suitable resources for the tree's growth and survival. The tree species may have had adaptations that allowed it to thrive in that particular environment.
The extinction of the tree species may have had cascading effects on other living things that were associated with it. For example, other plant species that relied on the tree for shade or as a host for epiphytic growth might have also suffered or disappeared. Similarly, animals that depended on the tree for food, shelter, or nesting sites could have been affected by its extinction.
Find out more on extinction here: https://brainly.com/question/1027555
#SPJ1
How many moles are present in 2.7 liters of Argon gas at STP?
Answers
6.9 cause argon is a proactive system that can be sick
Una especie que se aparea en la superficie y luego deposita sus huevos en el agua para ser rodeadas por los espermas, el de
Answers
The species would be an anuran (an amphibian)
Which type of species is it?We need to answer this in English.
Here we want to find "A species that mates on the surface and then deposits its eggs in the water to be surrounded by sperm"
This would be known as a species of amphibian called an anuran, specifically a frog or toad.
Frogs and toads belong to the order Anura and have a characteristic reproductive cycle that involves external fertilization. During reproduction, the male releases his sperm into the water, and the female deposits her eggs in the water as well. The eggs are externally fertilized by sperm that swim around them. This process is known as external or external fertilization.
Learn more about eggs:
https://brainly.com/question/1151355
#SPJ1
What is the difference between heat transfer and heat transformation
Answers
Answer:
heat transfer is when you transfer heat from one thing to another and heat transformation is when heat is transforming into something else hope it helps
Explanation:
Please someone help me ASAP I'm confused.

Answers
Help help with the question below

Answers
Select the answer with the correct number of significant figures for each calculation. 31.580 + 4.26 = 35.8 35.84 35.840
Answers
Answer:
35.8.540 its korrect now