Answers
The answer is B
Explanation:
Can you mark brainliest I correct
Related Questions
A reaction A(aq)+B(aq)↽−−⇀C(aq) A ( aq ) + B ( aq ) ↽ − − ⇀ C ( aq ) has a standard free‑energy change of −3. 06 kJ/mol − 3. 06 kJ / mol at 25 °C. 25 °C. What are the concentrations of A, A , B, B , and C C at equilibrium if, at the beginning of the reaction, their concentrations are 0. 30 M, 0. 30 M, 0. 40 M, 0. 40 M, and 0 M, 0 M, respectively?
Answers
At equilibrium, the concentrations of A, B, and C are 0.112 M, 0.212 M, and 0.188 M, respectively.
The standard free-energy change (ΔG°) of reaction is -3.06 kJ/mol, which can be related to equilibrium constant (K) using following equation:
ΔG° = -RTlnK
where R is gas constant (8.314 J/mol·K),
T is temperature in Kelvin (25°C = 298 K), and
ln is natural logarithm.
-3.06 kJ/mol = -8.314 J/mol·K x 298 K x lnK
Solving for lnK:
lnK = -3.06 kJ/mol / (-8.314 J/mol·K x 298 K) = 0.447
Taking, antilogarithm of both sides to solve for K:
K = e^0.447 = 1.563
At equilibrium, concentrations of A, B, and C can be calculated using stoichiometry of the reaction and equilibrium constant expression:
K = [C] / ([A] x [B])
1.563 = [C] / (0.30 M x 0.40 M)
[C] = 0.188 M
Since, stoichiometry of reaction is 1:1:1, [A] and [B] at equilibrium will be:
[A] = [A]0 - [C] = 0.30 M - 0.188 M = 0.112 M
[B] = [B]0 - [C] = 0.40 M - 0.188 M = 0.212 M
Therefore, at equilibrium, the concentrations of A, B, and C are 0.112 M, 0.212 M, and 0.188 M, respectively.
For more question on equilibrium click on
https://brainly.com/question/19340344
#SPJ4
1.) Nonmetallic elements form ions by (Losing/Gaining) valence electrons in order to complete their outer shell. 2.) The (Fewer/More) valence electrons an element has in its outer shell, the easier it is to complete. 3.) The (FewerMore) electron shells an element has, the easier it is to fill its outermost shell. 4&5.)Reactivity in nonmetals (Increases/Decreases) as you go from left to right in a group, and (Increases/Decreases) as you go from top to bottom.
Answers
Answer:
1) Gaining
2) More
3) More
4) increases
5) decreases
Explanation:
Nonmetals are found towards the right hand side of the periodic table. They form ions by gaining electrons due to the fact that they have more electrons in their outermost shell, hence they only need a few electrons to complete the octet structure.
As the number of shells increases, the outermost shell becomes more populated and easier to fill with electrons.
The reactivity of nonmetal elements generally increases from left to right across the periodic table but decreases from top to bottom down the group.
Answer:
1) Gaining
2) More
3) fewer
4) increases
5) decreases
Explanation:
A group of students working in a chemistry lab are planning a procedure to
neutralize hydrochloric acid (HCI, strong acid). How should they BEST accomplish
this?
Answers
use a strong base to neutralize, like NaOH, KOH, etc
What is the term that scientists use when comparing the
properties of solids, liquids and gases?
Answers
Answer:
Solids, liquids and gases are called the three states of matter. I think the answer is density. In general, solids are denser than liquids, which are denser than gases. Also solids have a fixed shape and a fixed volume. Liquids have a fixed volume, but no fixed shape. Gases have neither a fixed volume nor a fixed shape.
Explanation:
Hope this helps
Explain how nutrients are cycled in a food chain.
Answers
Answer:By their movement, by their wastes, and by their metabolic.
Explanation:The nutrient cycle is a system where energy and matter are transferred between living organisms and non-living parts of the environment. This occurs as animals and plants consume nutrients found in soil, and these nutrients are then released back into the environment via death and decomposition.
A nutrient cycle (or ecological recycling) is the movement and exchange of organic and inorganic matter back into the production of matter. Energy flow is a unidirectional and noncyclic pathway, whereas the movement of mineral nutrients is cyclic.
which of the following has no net dipole moment? n2o h2se teo3 nf3 ch3cl
Answers
The molecule that has no net dipole moment is TeO₃ (tellurium trioxide).
Let's analyze each of the molecules:
N₂O (nitrous oxide):
The molecule has a linear geometry with a N≡N triple bond. The bond dipoles do not cancel each other out, resulting in a net dipole moment. Therefore, N₂O has a net dipole moment.
H₂Se (hydrogen selenide):
The molecule has a bent or V-shaped geometry. The Se-H bonds have dipole moments, and due to the asymmetrical geometry, these dipole moments do not cancel each other out. Hence, H₂Se has a net dipole moment.
TeO₃ (tellurium trioxide):
The molecule has a trigonal planar geometry. The Te-O bonds have dipole moments, but they are arranged symmetrically around the central tellurium atom. The bond dipole moments cancel each other out, resulting in no net dipole moment for TeO₃.
NF₃ (nitrogen trifluoride):
The molecule has a trigonal pyramidal geometry. The N-F bonds have dipole moments, but again, due to the symmetrical arrangement of the bonds around the central nitrogen atom, the dipole moments cancel each other out. Thus, NF₃ has no net dipole moment.
CH₃Cl (Chloro-methane):
The molecule has a tetrahedral geometry. The C-Cl bond has a dipole moment, and since the molecule is asymmetrical, the dipole moments do not cancel each other out. Therefore, CH₃Cl has a net dipole moment.
Learn more about dipole moment here, https://brainly.com/question/11626115
#SPJ11
2C2H2(g) 5O2(g) → 4CO2(g) 2H2O(g) This is a balanced equation for the combustion of acetylene(C2H2). How many moles of oxygen(O2) are required to react completely with 1. 0 mole of acetylene(C2H2)?.
Answers
The moles of oxygen required to completely react with 1-mole acetylene is 2.5 mol.
The moles of reactant and product in a chemical reaction to the whole number ratio is given by the stoichiometric coefficient of the balanced chemical equation.
Computation for the moles of oxygenThe balanced chemical equation for the reaction is :
\(\rm 2\;C_2H_2\;+\;5\;O_2\;\to\;4\;CO_2\;+\;2\;H_2O\)
From the balanced chemical equation, the 2 moles of acetylene react with 5 moles of oxygen.
The moles of oxygen react with 1 mole of acetylene are:
\(\rm 2\;mol\;C_H_2=5\;mol\;O_2\\\\1\;mol\;C_2H_2=\dfrac{5}{2}\;\times\;1\;mol\;O_2\\\\ 1\;mol\;C_2H_2=2.5\;mol\;O_2\)
The moles of oxygen required to completely react with 1-mole acetylene is 2.5 mol.
Learn more about moles reacted, here:
https://brainly.com/question/24817060
Copper can make a +2 ion, and if it does, what will be the formula for
copper sulfate?
Answers
Answer:
CuSO4
A metal sulfate compound having copper(2+) as the metal ion. This entity has been manually annotated by the ChEBI Team. Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula CuSO4
What is the chemical formula for 8.6 mol of sulfur and 3.42 mol of phosphorus
Answers
The chemical formula for the compound containing 8.6 mol of sulfur and 3.42 mol of phosphorus is P₂S₅
How do I determine the formula of the compound?From the question given above, the following data were obatined:
Sulphur (S) = 8.6 molesPhosphorus (P) = 3.42 moleChemical formula =?The chemical formula of the compound can be obtained as follow:
Divide by their molar mass
S = 8.6 / 32 = 0.26875
P = 3.42 / 31 = 0.11032
Divide by the smallest
S = 0.26875 / 0.11032 = 2.44
P = 0.11032 / 0.11032 = 1
Multiply by 2 to express in whole number
S = 2.44 × 2 = 5
P = 1 × 2 = 2
Thus, the chemical formula is P₂S₅
Learn more about empirical formula:
https://brainly.com/question/9459553
#SPJ1
what is hydraulic pressure?
Answers
Calculate the wave length of hello light emitted by sodium lamp if the frequency of the radiation is 5x10to the power of 4
Answers
6.0×10⁵m is the wavelength of hello light emitted by sodium lamp if the frequency of the radiation is 5x10⁴.
What is wavelength?A periodic wave's wavelength is its spatial period, or the length over which its form repeats. It is a property of the both traveling waves as well as standing waves in addition to different spatial wave patterns. It refers to the distance between two successive corresponding locations of the same phase just on wave, such as two nearby crests, troughs, and zero crossings.
The spatial frequency is the reciprocal of wavelength. The Greek letter lambda is frequently used to represent wavelength. The term wavelength also was occasionally used to refer to modulated waves, their sinusoidal envelopes, or waves created by the interaction of several sinusoids.
ν=c/λ
5x10⁴=3×10⁸/λ
λ =3×10⁸/ 5x10⁴
=6.0×10⁵m
Therefore, 6.0×10⁵m is the wavelength.
To know more about wavelength, here:
https://brainly.com/question/12377285
#SPJ9
In lab students are going to burn strips of magnesium. If oxygen is needed to burn the magnesium in a synthesis reaction, what would this chemical equation be
Answers
The chemical equation for the synthesis reaction of burning magnesium in the presence of oxygen can be summarized as: 2 Mg + O2 → 2 MgO
In this reaction, magnesium (Mg) reacts with oxygen (O2) to form magnesium oxide (MgO). The balanced equation shows that two moles of magnesium react with one mole of oxygen to produce two moles of magnesium oxide.
When magnesium is burned, it undergoes a redox reaction with oxygen. Magnesium atoms lose two electrons to form Mg2+ ions, while oxygen molecules gain four electrons to form O2- ions.
The resulting ions combine to form the ionic compound magnesium oxide (MgO), which is a white solid. The balanced equation reflects the stoichiometry of the reaction, indicating the correct ratio of reactants and products.
To learn more about chemical equation click here: brainly.com/question/28792948
#SPJ11
How many moles are in 3.59 x 10^10 kg of gallium ?

Answers
if i’m wrong someone correct me please
Which species in each pair has the greater polarizability?
Na+ or Na
CH3COOH or CH3CH2COOH
BCl3 or BF3
Answers
Na has greater polarizability than Na+.
CH3CH2COOH has greater polarizability than CH3COOH.
BCl3 has greater polarizability than BF3.
Polarizability refers to the ability of an atom or molecule to undergo distortion of its electron cloud in the presence of an electric field. Generally, larger atoms or molecules with more diffuse electron clouds have greater polarizability.
Na+ or Na: Na+ is a cation, which means it has lost an electron compared to the neutral Na atom. Since Na+ has a smaller electron cloud, it has lower polarizability compared to Na.
CH3COOH or CH3CH2COOH: CH3CH2COOH (acetic acid) has greater polarizability than CH3COOH (ethyl acetate). The additional ethyl group in CH3CH2COOH increases the size and electron cloud of the molecule, making it more polarizable.
BCl3 or BF3: BCl3 (boron trichloride) has greater polarizability than BF3 (boron trifluoride). Chlorine (Cl) atoms are larger than fluorine (F) atoms, resulting in a larger electron cloud and higher polarizability for BCl3 compared to BF3.
Na has greater polarizability than Na+. CH3CH2COOH has greater polarizability than CH3COOH. BCl3 has greater polarizability than BF3.
To learn more about Polarizability , visit
brainly.com/question/31112089
#SPJ11
8. how many joules are required to heat 55.8 g of tin from 36.4 degrees * c to 7.7 degrees * c (c=0.213 j/g*^ c)
Answers
The amount of heat required to heat 55.8g of tin from 36.4°C to 7.7°C is -311.5 Joules.
The amount of heat required to heat 55.8g of tin from 36.4°C to 7.7°C can be calculated using the formula q = m × c × ΔT,
where q represents the heat, m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature.
First, we find the change in temperature by subtracting the initial temperature from the final temperature: ΔT = 7.7°C - 36.4°C = -28.7°C.
Next, we convert the mass of tin to grams: Mass of tin = 55.8g.
Using the formula, we calculate the amount of heat: q = 55.8g × 0.213 J/g °C × (-28.7°C) = -311.5 J.
The negative sign indicates that heat was removed from the tin during the process of cooling it down.
Therefore, the amount of heat required to heat 55.8g of tin from 36.4°C to 7.7°C is -311.5 Joules.
To know more about Heat Energy here: brainly.com/question/934320
#SPJ11
Which of the following examples is evidence of a physical change?
A: A person inhales oxygen and exhales carbon dioxide and water.
B:Ice cream melts in a bowl.
C:A silver spoon tarnishes over time.
D:An electrical current splits water into hydrogen and oxygen.
Answers
In the given examples ice cream melts in a bowl is an example of a physical change.
A physical change occurs when a substance transforms from one state to another i.e., the change only affects its physical properties like color, volume, shape, and phase changes.
Using water as an example, if we have it at room temperature, it is a clear liquid. If we decrease the temperature below the freezing point, the water freezes and forms ice. If we increase the temperature above the boiling point, the water turns into a gas(steam). We can see that in all three forms, the water is still composed of two hydrogen atoms and one oxygen atom. Nothing is added or subtracted from it, which means the chemical composition stays the same. The transformation of water from liquid to solid or gas is an example of a physical change.
Learn more about physical change here:
https://brainly.com/question/13339068
which is not a correct ion?
li2+
cl-
Al3
O2-
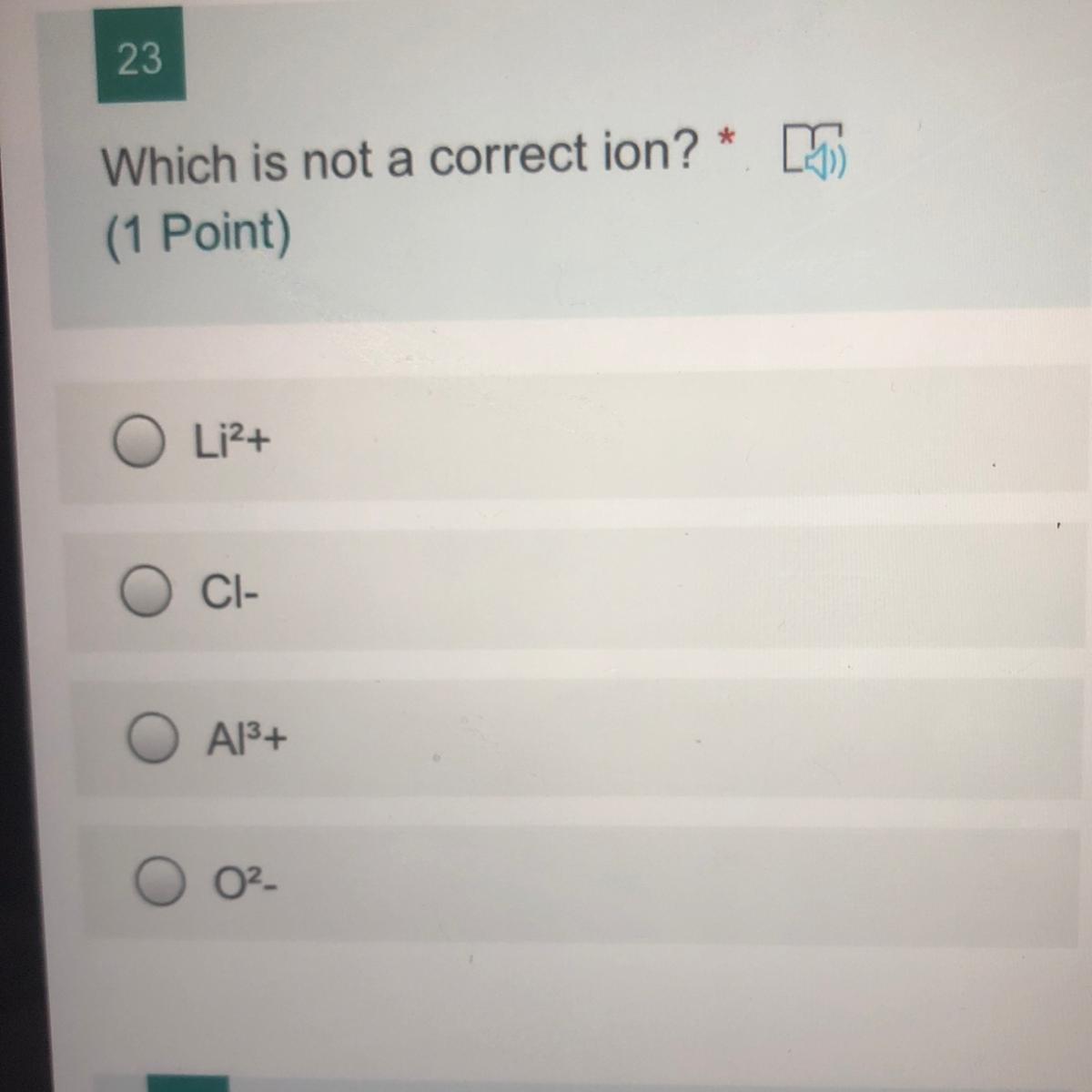
Answers
Answer:
The answer is option 1.
Explanation:
Lithium is a Group 1 element. So the correct charge for lithium should be Li+.
Answer:
(a)\(Li^{2+}\)
Explanation:
Answer is (a)\(Li^{2+}\)
As lithium has only one valence electron, it will lose one electron to form a cation with charge 1+ . Thus the correct Li ion is \(Li^{1+}\)
The specific type of bond that results from unequal sharing of electrons in the bond is
Answers
Answer:
It's called A polar covalent bond
How many moles are in 5.3 X 10^6 atoms of Calcium (Ca)?
Answers
Answer:
8.801×10^-18 moles Ca
Explanation:
5.3 X 10^6atoms* (1mol/6.022*10^23) = 8.801×10^-18 moles Ca
what is the common name of this compound? group of answer choices 3-cyclopentylethanol 1-cyclopropyl-1-ethylalcohol ethylcyclopentanol cyclopentyl ethyl ether cyclopentyl ethyl ketone
Answers
The common name of the compound is 3-cyclopentylethanol.
Based on the given answer choices, the common name of the compound 1-cyclopropyl-1-ethylalcohol is ethylcyclopentanol. Here's a breakdown of the terms:
1. 3-cyclopentylethanol: This refers to an alcohol with an ethyl group (2 carbons) and a cyclopentyl group (5 carbon ring) connected to the third carbon of the ethyl group.
2. 1-cyclopropyl-1-ethylalcohol (ethylcyclopentanol): This compound has a cyclopropyl group (3 carbon ring) and an ethyl group connected to the same carbon, resulting in the common name ethylcyclopentanol.
3. Cyclopentyl ethyl ether: This compound is an ether with a cyclopentyl group and an ethyl group connected by an oxygen atom.
4. Cyclopentyl ethyl ketone: This compound is a ketone with a cyclopentyl group and an ethyl group connected by a carbonyl group (C=O).
So, the common name of the compound 1-cyclopropyl-1-ethylalcohol is ethylcyclopentanol.
To know more about cyclopentyl visit:
https://brainly.com/question/12621202
#SPJ11
True or False: Only animals can move pollen from plant to plant.
A. True
B. False
Answers
i think false because of the wind or insects
found to have a mass of 37.16 grams. brass is an alloy composed of only copper and zinc, and it reacts with an excess amount of 86.203 grams of hydrochloric acid via the balanced equation below. at the end of the reaction, 45.387 grams of zinc chloride was produced. what was the mass of hydrogen gas formed
Answers
According to the balanced equation, zinc metal interacts with hydrochloric acid: ZnCl2(aq) + H2 Zn(s) + 2 HCl(aq) ( g) In a coffee-cup calorimeter 0.103 g of Zn(s) are mixed with 50.0 mL of HCl to create a solution. All of the zinc reacts, increasing the solution's temperature from 22.5 °C to 23.7 °C. To perform this reaction as described, locate Hrxn(Use 4.18 J/g # °C as the specific heat capacity and 1.0g mL as the density of the solution
Explanation: Before doing the arithmetic use "Dimensional Analysis to establish what conversion factors you will need. g \smL \s× \s?? \s?? \s= \slbs \sgal
We require a volume conversion unit (mL gal) and a mass conversion unit (g pounds)For the sake of our calculation 1 lb equals 454 g
Volume will be calculated in two steps: m L L and t h e n L g a l
1 g a l equals 3.785 L and 1 L equals 1000 m LIf we combine these we get
1 \sg \sa \sl \s= \s3.785 \sL \s× \s1000 \sm \sL \sL \s= \s3785 \sm \sL
3785 \sm \sL \s1 \sg \sa \sl
Learn more about 1.0g mL here:
https://brainly.com/question/17441842
#SPJ4
An oven takes in 1200J of energy
and transfers 375J as useful energy.
Caculate the energy.
Answers
The energy is 375 J.
Energy is the quantitative belongings that are transferred to a frame or to a physical gadget, recognizable within the overall performance of labor and within the shape of warmth and mild. power is a conserved amount—the law of conservation of strength states that power may be converted in form, but now not created or destroyed.
Calculation:-
Total energy = 1200 J
Transfered emergy = 375 J
The useful energy is the energy to do work = 375 J.
The ability or strength to do paintings, along with the potential to transport an item (of a given mass) by means of the application of pressure. power can exist in a spread of forms, which includes electric, mechanical, chemical, thermal, or nuclear, and may be transformed from one form to another.
Learn more about energy here:-https://brainly.com/question/13881533
#SPJ1
Which type of reaction occurs in the following?
2HgO(s) Right arrow. 2Hg(l) + O2(g)
a decomposition reaction in which a metal is reduced
an acid-base neutralization reaction to produce oxygen gas
a displacement reaction involving two metals
a combustion reaction involving burning a metal in oxygen
Answers
Answer:
decomposition
Explanation:
the reactants turn into two different products, thus the reaction is decomposition.
AB -> A + B
Answer:
A: a decomposition reaction in which a metal is reduced
Explanation:
HgO is actually the metal Mercury and in the equation Mercury is being decomposed into the elements Hg and O. (the element for Mercury and Oxygen) So the equation they give you is a decomposition.

Propane (C3H8), a common fuel, reacts with oxygen to form carbon dioxide and water according to the equation below: C3H8 + 5O2 → 3CO2 + 4H2O If a propane heater burns 38.95 g C3H8, it consumes
Answers
Answer:
0.8833 mole of C3H8
Explanation:
Given:
Mass of C₃H₈ (Propane) = 38.95 gram
Computation:
Molar mass of C = 12.01
Molar mass of H = 1.008
Molar mass of C₃H₈ (Propane) = 3(12.01) + 8(1.008)
Molar mass of C₃H₈ (Propane) = 36.03 + 8.064
Molar mass of C₃H₈ (Propane) = 44.094
Number of mol in 38.95 gram Propane = Mass / Molar mass
Number of mol in 38.95 gram Propane = 38.95 / 44.094
Number of mol in 38.95 gram Propane = 0.88334
Answer:
Use the Periodic Table to find molar masses.
Propane (C3H8), a common fuel, reacts with oxygen to form carbon dioxide and water according to the equation below:
C3H8 + 5O2 → 3CO2 + 4H2O
If a propane heater burns 38.95 g C3H8, it consumes
38.95 mol C3H8.
0.8830 mol C3H8.
1 mol C3H8.
44.10 mol C3H8.
Part 2
How many moles of oxygen are required to produce 37.15 g CO2?
37.15 g CO2 = 1.407 mol O2
Part 3
What mass of propane is necessary to react with the amount of oxygen calculated in the previous question?
12.41 g C3H8
What mass of magnesium sulfate is dissolved in 400. mL of a 0.800 M solution of magnesium sulfate in water
Answers
Therefore, the mass of magnesium sulfate dissolved in 400 mL of the 0.800 M solution is 38.47 grams.mass = concentration (M) × volume (L) × molar mass (g/mol)
Given:
The concentration of magnesium sulfate solution = 0.800 M
Volume of solution = 400 mL = 400 mL × (1 L / 1000 mL) = 0.4 L
The molar mass of magnesium sulfate (MgSO4) is:
Mg: 24.31 g/mol
S: 32.06 g/mol
O (4 × 16.00): 64.00 g/mol
Molar mass of MgSO4 = 24.31 + 32.06 + 64.00 = 120.37 g/mol
Plugging the values into the formula:
mass = 0.800 M × 0.4 L × 120.37 g/mol
mass = 38.47 g
Learn more about the mass of magnesium sulfate here:
https://brainly.com/question/13412525
#SPJ11
How many moles of nitrogen dioxide gas will be produced if 25.0 ml of 2M nitric acid react?
Cu + HNO3 = Cu(NO3)2 + NO2 + H2O
Answers
Answer:
0.025 moles of NO₂ will produced
Explanation:
Given data:
Moles of NO₂ formed = ?
Volume of HNO₃ = 25.0 mL
Molarity of HNO₃ = 2 M
Solution:
Chemical equation:
Cu + 4HNO₃ → Cu(NO₃)₂ + 2NO₂ + 2H₂O
Number of moles of HNO₃:
Molarity = number of moles / volume in L
2M = number of moles / 0.025 L
Number of moles = 2 M × 0.025 L
Number of moles = 0.05 mol
Now we will compare the moles of HNO₃ with NO₂ from balance chemical equation.
HNO₃ : NO₂
4 : 2
0.05 : 2/4×0.05 =0.025
0.025 moles of NO₂ will produced.
A liquid in the lab has a density of 1.24 g/cm . what is the mass in grams of 254
ml of the liquid?
please show the work as well
thank you
Answers
Answer: 315 g
Explanation:
\(d=\frac{m}{v} \implies m=dv=(254 \text{ mL})(1.24 \text{ g/cm})=315 \text{ g (to 3 sf)}\)
In the given question, 314.96 g is the mass of 254 ml of the liquid that is used in lab and has density of 1.24 \(\rm g/cm^3\).
Mass is the amount of substance in a thing is determined by mass, which is a characteristic of matter. It is usually expressed in grams (g) or kilograms (kg).
To find the mass, we can use the formula:
mass = volume x density
where the volume is given as 254 mL and the density is given as1.24 \(\rm g/cm^3\)
Substituting the given values, we get:
mass = 1.24 g/cm³ \(\times\) 254 ml
Before we can calculate the mass, we need to convert milliliters (ml) to cubic centimeters (cm³), since the density is given in g/cm³. 1 ml is equivalent to 1 cm³, so:
mass = 1.24 g/cm³ \(\times\) 254 cm³
mass = 314.96 g
Therefore, the mass of 254 ml of the liquid is 314.96 g.
Learn more about mass here:
https://brainly.com/question/11954533
#SPJ4
How do particles move when a transverse wave passes through a medium?
A. In wavelengths and amplitudes
B. Up to a crest, then down through a trough, then back
C. Through a compression followed by a rarefaction
D. Along circular or elliptical paths
Answers
Answer: In a transverse wave, particles of the medium vibrate up and down perpendicular to the direction of the wave. ... In a surface wave, particles of the medium vibrate both up and down and back and forth, so they end up moving in a circle.
Explanation:
Hope this helps :)
Calculate the pH during a titration when 9.54 mL of a 0.15 M HCl solution has reacted with 22.88 mL of 0.14 M NaOH?
Answers
We can use the fact that Kw = [H+][OH-] = 1.0 x 10^-14 at 25°C to calculate the concentration of hydrogen ions ([H+]) in the solution: [H+] = Kw / [OH-] = (1.0 x 10^-14) / (0.0626) = 1.60 x 10^-13 M The pH of the solution is: pH = -log[H+] = -log(1.60 x 10^-13) = 12.80. The balanced equation for the reaction between HCl and NaOH is: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
First, we need to determine the number of moles of HCl and NaOH used in the reaction:
moles of HCl = 0.15 M x 9.54 mL / 1000 mL = 0.001431 moles
moles of NaOH = 0.14 M x 22.88 mL / 1000 mL = 0.003203 moles
Next, we need to determine which reactant is the limiting reagent. From the balanced equation, we can see that 1 mole of HCl reacts with 1 mole of NaOH. Therefore, HCl is the limiting reagent because it has fewer moles than NaOH.
The number of moles of HCl that reacted is equal to the number of moles of NaOH that reacted because they react in a 1:1 stoichiometric ratio. Therefore, 0.001431 moles of HCl reacted.
The total volume of the solution after the reaction is:
V = VHCl + VNaOH = 9.54 mL + 22.88 mL = 32.42 mL = 0.03242 L
The concentration of the remaining NaOH can be calculated using the following equation:
MNaOH = moles of NaOH / V
MNaOH = (0.003203 moles - 0.001431 moles) / 0.03242 L = 0.0626 M
Now we can use the fact that NaOH is a strong base and completely dissociates in water to calculate the concentration of hydroxide ions ([OH-]) in the solution:
[OH-] = MNaOH = 0.0626 M
Finally, we can use the fact that Kw = [H+][OH-] = 1.0 x 10^-14 at 25°C to calculate the concentration of hydrogen ions ([H+]) in the solution:
[H+] = Kw / [OH-] = (1.0 x 10^-14) / (0.0626) = 1.60 x 10^-13 M
Therefore, the pH of the solution is:
pH = -log[H+] = -log(1.60 x 10^-13) = 12.80
Learn more about HCl here:
https://brainly.com/question/16727708
#SPJ11
