Select the correct statements about an aqueous amphoteric salt solution. it?s always basic the pH depends upon the concentration of the salt it?s always acidic it?s always neutral because it is a salt it can be basic or acidic depending upon the pKa values the pH depends only on the salt species not on concentration
Answers
The correct statements about an aqueous amphoteric salt solution are 'the pH depends upon the concentration of the salt' and 'it can be basic or acidic depending upon the pKa values'.
An amphoteric salt solution is a unique type of solution because it has the ability to act as either an acid or a base, depending on its surrounding conditions. The behavior of an aqueous amphoteric salt solution is determined by the pKa values of the acidic and basic components, which ultimately define its acidity or basicity.
1. The pH depends upon the concentration of the salt: The concentration of the salt influences the ratio of acidic and basic species in the solution, which in turn affects the pH.
2. It can be basic or acidic depending upon the pKa values: The pKa values of the acidic and basic components in the amphoteric salt determine whether the solution will behave as an acid or a base. If the pKa of the acidic component is lower than that of the basic component, the solution will be acidic.
The incorrect statements about an amphoteric salt solution are: it's always basic, it's always acidic, it's always neutral because it is a salt: While many salts do produce neutral solutions, amphoteric salts can produce solutions that are acidic or basic, depending on the pKa values and concentrations, the pH depends only on the salt species, not on concentration.
Know more about amphoteric salt here:
https://brainly.com/question/29098795
#SPJ11
Related Questions
how many electrons each atom gives or takes in KBr
Answers
A chemistry class is reviewing an analysis of particles present during nuclear fusion reactions between hydrogen isotopes deuterium and tritium. The students were asked to use the model and data below to explain what occurs during fusion.

Answers
A chemistry class is reviewing an analysis of particles present during nuclear fusion reactions between hydrogen isotopes deuterium and tritium. the students were asked to use the model and data below to explain occurs during fusion then the fusion can be supported by the data is two smaller atoms come together to form a larger atom that is the same element
Fusion is the process in which two atom slam together to form the heavier atom called as fusion
And here according to the given data particle present before fusion in deuterium mass is two and in tritium mass is three and particle present after fusion in helium mass is four and neutron mass is one so according to the data of fusion two smaller atoms come together to form a larger atom that is the same element
Know more about nuclear fusion reactions
https://brainly.com/question/9054784
#SPJ1
Maria owns a bakery and bakes many different types of yeast bread. One day she notices that the bread she baked is flat and does not contain the air bubbles that are usually present. Which of the following would be a good explanation for what has happened to her yeast? (A) the yeast produced too much carbon dioxid. (B) the yeast did not produce the alcohol needed for the bread to rise. (C) the yeast were very active and produced too much energy. (D)the yeast did not produce the carbon dioxide needed for the bread to rise.
Answers
The bubbles in the bread is caused by the evolution of carbon dioxide by the action of yeast. If there are no bubbles formed inside the bread which means, the yeast did not produce the carbon dioxide needed for the bread to rise.
What is fermentation ?Fermentation is the process of conversion of sugar molecule into other substance b the action of microbes such as yeast. In cellular respiration the glucose molecule is converting to carbon dioxide and water. Where as, in fermentation, the glucose is converting to carbon dioxide and ethanol.
The balanced chemical equation of the breaking of glucose into carbon dioxide and water is written as follows:
\(\rm C_{6}H_{12}O_{6} \rightarrow 2CO_{2} + 2C_{2}H_{5}OH.\)
According to this reaction, one mole of glucose molecule gives 2 moles of carbon dioxide and 2 moles of ethanol. Hence, if the bread do not rise the bubbles, the yeast does not producing CO₂.
Find more on fermentation :
brainly.com/question/14525128
#SPJ1
Consider the reaction. 2 upper H upper F (g) double-headed arrow upper H subscript 2 (g) plus upper F subscript 2 (g). At equilibrium at 600 K, the concentrations are as follows. [HF] = 5. 82 x 10-2 M [H2] = 8. 4 x 10-3 M [F2] = 8. 4 x 10-3 M What is the value of Keq for the reaction expressed in scientific notation? 2. 1 x 10-2 2. 1 x 102 1. 2 x 103 1. 2 x 10-3.
Answers
The value of the equilibrium constant for the dissociation of HF is \(\rm 1.2\;\times\;10^-^3\). Thus, option D is correct.
The given balanced chemical equation is:
\(\rm 2\;HF\;\leftrightharpoons H_2\;+\;F_2\)
The equilibrium constant for the reaction is given as the ratio of the concentration of product to reactant raised to the stoichiometric coefficient.
Computation of equilibrium constantThe equilibrium constant (Keq) for the given reaction is given as:
\(Keq=\rm \dfrac{[H_2]\;[F_2]}{[HF]^2}\)
The equilibrium concentration is given as:
\(\rm HF=5.82\;\times\;10^-^2\;M\)\(\rm H_2=8.4\;\times\;10^-^3\;M\)\(\rm F_2=8.4\;\times\;10^-^3\;M\)Substituting the values for the equilibrium constant:
\(Keq=\dfrac{[8.4\;\times\;10^-^3]\;[8.4\;\times\;10^-^3]}{[5.82\;\times\;10^-^2]} \\\\Keq=12.1\;\times\;10^-^4\\\\Keq=1.2\;\times\;10^-^3\)
The value of the equilibrium constant for the dissociation of HF is \(\rm 1.2\;\times\;10^-^3\). Thus, option D is correct.
Learn more about the equilibrium constant, here:
https://brainly.com/question/17960050
Matter can not be created nor destroyed: it can only be
a)Destroyed a little bit
b)Invisible
c)Transformed, changed
d)None of the above
Answers
Answer:
C- transforemed or changed
Explanation:
matter can be changed by application of heat or cold
The video shows the addition of aqueous sodium chloride to a solution of aqueous silver nitrate.
Write the balanced molecular equation for the reaction. Include phases.

Answers
The balanced molecular equation for the reaction would be: \(NaCl (aq) + AgNO_3 (aq) --- > NaNO_3 (aq) + AgCl (aq)\)
Balanced equation of reactionsA balanced molecular equation is an equation that shows the molecular formulas of the reactants and products of reactions in agreement with the law of conservation of atoms.
Aqueous sodium chloride reacts with aqueous silver nitrate to produce aqueous sodium nitrate and silver nitrate precipitates.
Formula for sodium chloride = NaCl
Formula for silver nitrate = AgNO3
Formula for silver chloride = AgCl
Formula for sodium nitrate = NaNO3
Overall balanced equation of the reaction: \(NaCl (aq) + AgNO_3 (aq) --- > NaNO_3 (aq) + AgCl (aq)\)
More on balanced equations can be found here: https://brainly.com/question/29109946
#SPJ1
Help please! A gas occupies 500 mL at 270 mm Hg and 55°C. If the pressure is changed to 1.8 atm and the temperature is
increased to 100°C, what is the new volume? Be sure to show the setup and the final answer and unit.
Show Your Work
Answers
Answer:
V₂ = 112.14 mL
Explanation:
Given data:
Initial volume = 500 mL
Initial pressure = 270 mmHg (270/760 =0.355 atm)
Initial temperature = 55 °C (55 +273 = 328 K)
Final temperature = 100°C (100+273 = 373 K)
Final volume = ?
Final pressure = 1.8 atm
Formula:
P₁V₁/T₁ = P₂V₂/T₂
P₁ = Initial pressure
V₁ = Initial volume
T₁ = Initial temperature
P₂ = Final pressure
V₂ = Final volume
T₂ = Final temperature
Solution:
V₂ = P₁V₁ T₂/ T₁ P₂
V₂ = 0.355 atm × 500mL × 373 K / 328 K × 1.8 atm
V₂ = 66207.5 atm .mL. K / 590.4 K.atm
V₂ = 112.14 mL
which of the given purposes is the tlc method most often used for?
Answers
Thin layer chromatography (TLC) is an affinity derived approach that works to separate compounds in a mixture. TLC is deeply versatile separation method used for both qualitative and quantitative sample analysis.
In general, Thin layer chromatography is performed by using a thin, uniform layer of silica gel or alumina coated onto a piece of glass, metal or rigid plastic. These silica gel is present in stationary phase. There are three most important industrial applications that thin layer chromatography has are clinical, pharmaceutical, and food testing approaches. One of the most common specific types of clinical tests performed with TLC is for the presence of drugs of abuse.
To learn more about Thin layer chromatography , here
brainly.com/question/10296715
#SPJ4
Atoms of a given elements with have the same mass true or false?
Answers
Answer: True
Explanation:
what is the decay constant for carbon-10 if it has a half-life of 19.3s? what is the decay constant for carbon-10 if it has a half-life of 19.3s?
A. 27.8/s B. 0.0518/s
C. 0.0359/s D. 13.4s
Answers
The decay constant (λ) can be calculated using the half-life (t½) of a radioactive substance using the following formula:
λ = ln(2) / t½
Given that the half-life of carbon-10 is 19.3 seconds, we can calculate the decay constant as follows:
λ = ln(2) / 19.3
Using a calculator, we find that λ is approximately 0.0359/s.
Therefore, the correct answer is:
C. 0.0359/s
To know more about radioactive refer here
https://brainly.com/question/1770619#
#SPJ11
A solid form of matter in which there is long range repeating order is called ________
Answers
Answer: A solid form of matter in which there is long-range repeating order is called crystalline.
Explanation:
A solid is crystalline if it has long-range order. Once the positions of an atom and its neighbors are known at one point, the place of each atom is known precisely throughout the crystal.
2. A tow-line with a tension of 1200 N at 35° is needed to tow the 1350 kg
trailer at a constant speed. What is the uk?
Answers
Answer:
i don't know
sorry i would help but i don't know
Explanation:
How many moles of magnesium is 1. 25
x 1023 atoms of magnesium?
Answers
1.25 x 1023 atoms of magnesium contains 0.28. moles of magnesium.
A mole of any element contains Avogadro's number of that element's atoms. To find the number of moles of magnesium in 1.25 x 1023 atoms of magnesium, simply divide by Avogadro's number:
1.25 x
\( {10}^{23} \)
magnesium atoms / 6.022 x
\( {10}^{23} \)
atoms/mol = 0.208 moles magnesium
As a result, 1.25 x
\( {10}^{23} \)
atoms of magnesium contain 0.208 moles of magnesium.
The number of particles (atoms, molecules, and ions) in one mole of a substance is represented by Avogadro's number, which is a fundamental constant in chemistry. It has a value of about 6.022 x
\( {10}^{23} \)
particles per mole. The number was named after Italian scientist Amedeo Avogadro, who first proposed the concept in the early 19th century as a way to explain the relationship between the amount of a substance and its constituent particles.
learn more about moles here:
https://brainly.com/question/28239680
#SPJ4
LOTS OF POINT PLS HELP!!!! What is the balanced, net ionic equation for the reaction shown below? HCI (aq) + NaOH (ag)-> NaCI (aq) +H2P(l)


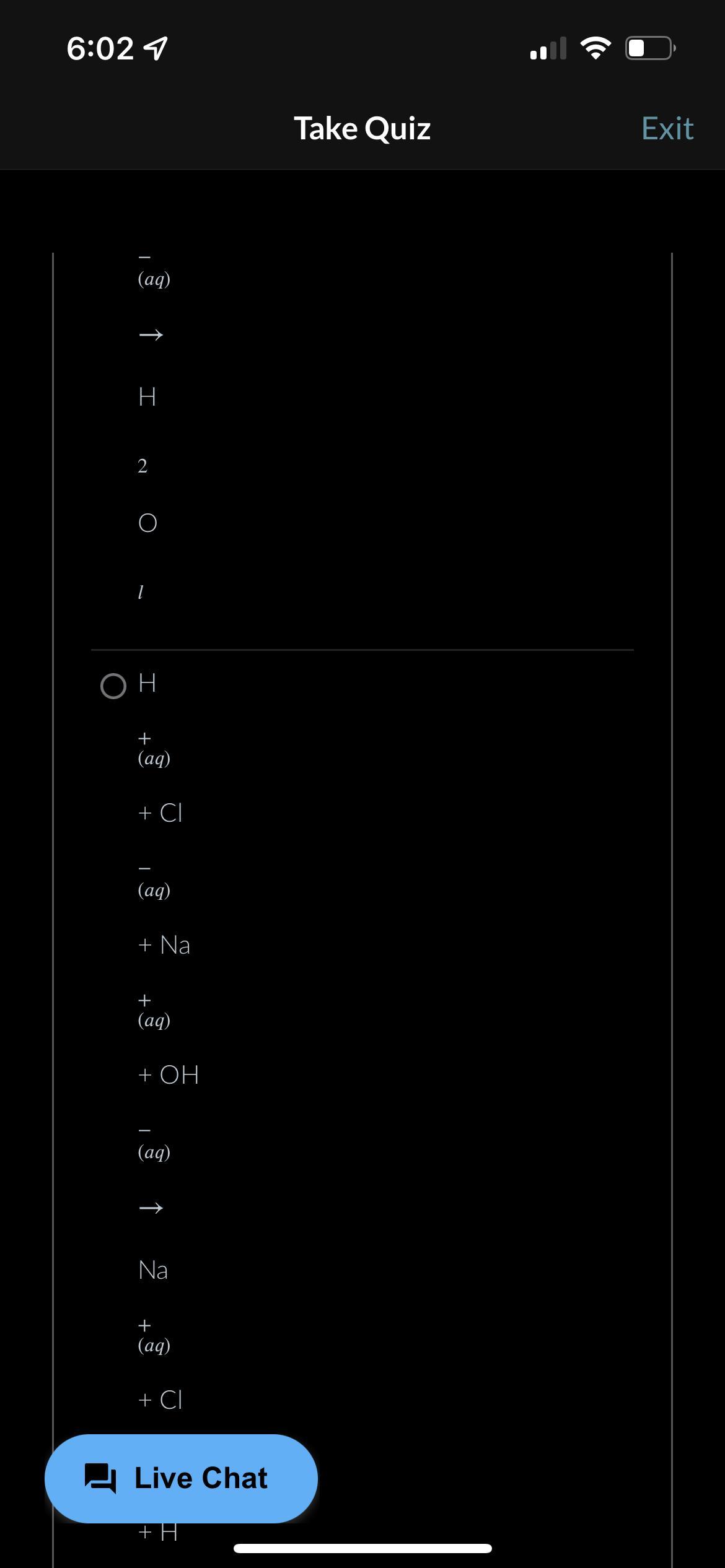

Answers
The net ionic equation is written as H^+(aq) + OH^-(aq) -------> H2O(l)
What is the net ionic equation?The term net ionic equation refers to the equation that shows the ions that underwent a change in the reaction. We have to note that the reaction species here must be ionic species which are able to dissociate into ions in solutions.
Now the first step is to put down the molecular equation. The molecular equation shows the reaction of the compounds as follows;
HCI (aq) + NaOH (ag)-> NaCI (aq) +H2O(l)
Next, we put own the complete ionic reaction equation as follows;
H^+(aq) + Cl^-(aq) + Na^+(aq) + OH^-(aq) -------> Na^+(aq) + + Cl^-(aq) + H2O(l)
Next we have the net ionic equation;
H^+(aq) + OH^-(aq) -------> H2O(l)
Learn more about ionic reaction equation:https://brainly.com/question/21368817
#SPJ1
A recipe calls for 3 tablespoons of milk for 7 pancakes. If this recipe was used to make 28 pancakes, how many tablespoons of milk would be needed
A. 15
B. 11
C. 12
D. 9
Answers
The number of tablespoons of milk needed for 28 pancakes is determined as 12 tablespoons.
option C is the correct answer.
How many tablespoons of milk would be needed?The number of the tablespoons of milk that would be needed is calculated by applying simple proportion method.
3 tablespoons of milk for 7 pancakes;
3 -----------> 7
? tablespoons of milk for 28 pancakes;
? --------------------> 28
Combine the two equations and solve for the number of tablespoons needed as follows;
? = ( 3 x 28 ) / 7
? = 12
Thus, The number of tablespoons of milk needed for 28 pancakes is determined by applying simple proportion.
Learn more about proportion here: https://brainly.com/question/19994681
#SPJ1
during photosynthesis, light energy from the Sun causes a reaction to take place. The products of photosynthesis are used in a reaction during the process of cellular respiration, which provides energy for cell processes.
Identify which reaction absorbs energy and which reaction releases energy.
Answers
Answer:
chemical reactions which proceed with the release of heat energy are called exothermic reactions
What is the molarity of a 50.0ml aqueous solution containing 10.0 grams of hydrogen peroxide H2O2
Answers
Molarity= No of moles of solute * 1000 / vol solution in ml
No of moles= Given mass / Molar mass
Given Mass of solute (H2O2)= 10g
Molar mass of H2O2=34gmol^-1
No of moles= 10/34= 0.294 moles
Volume of solution=50ml
Molarity = 0.294*1000 / 50
Molarity = 5.8M
what product of an acid base reaction is an ionic compound
A. water
B. a metal
C. a gas
D. a salt
Answers
The product of an acid-base reaction that is most likely to be an ionic compound is a salt. Option D).
In an acid-base reaction, the product that is most likely to be an ionic compound is a salt. A salt is formed when an acid reacts with a base, resulting in the transfer of ions between the two reactants. Acids typically release hydrogen ions (H+) when dissolved in water, while bases release hydroxide ions (OH-). When these ions combine, they form water (H2O), which is a neutral molecule.
However, in addition to water, the reaction between an acid and a base also produces a salt. A salt is an ionic compound composed of positive and negative ions. The positive ion usually comes from the base, while the negative ion comes from the acid. The combination of these ions results in the formation of an ionic compound, which is commonly referred to as a salt.
Therefore, the product of an acid-base reaction that is most likely to be an ionic compound is a salt. Hence option D) is correct.
For more question on salt
https://brainly.com/question/20835655
#SPJ8
determine the enthalpy change when 7.08g of Ag is obtained. use the abbreviated form of the unit in your answer.
2AgBr(s) -> 2Ag(s) + Br2(l) deltaH = -220kJ
Answers
2AgBr(s) ⇒ 2Ag(s) + Br₂(l) ΔH = -220kJ ⇒ for 2 mol Ag
mol Ag = 7.08 : 108 g/mol = 0.065
the enthalpy change = 0.065/2 x -220 kJ = -7.15 kJ
aluminum metal (27.0 g/mol) is formed by the electrolysis of molten al2o3. how many grams of al are produced when 5 ×103 coulombs pass through the cell?
Answers
AL are produced when 5 ×103 coulombs pass through the cell: 3. 0.243 grams
1 mol e− carries a charge of 1 Faraday
and 1 Faraday = 96485 Coulombs.
Then
No of moles of e− in the electrolysis:
2.6 x 10^3 C / 96485 C. mol^-1
= 0.0269 moles of electrons
From the reduction equation, we know that, 3 moles of e- are needed to reduce 1 mol of Al3+ to Al metal.
Therefor, the mole of Al to be deposited (Cathode) are
0.02695 / 3 = 0.00898 mol of Al
Molar mass of Al = 27 gram/mol
Mass of Al produced = 0.00898 mol * 27 gram/ mol = 0.243 grams
Electrolysis may be used to split a substance into its authentic additives/factors and it become thru this manner that some of factors have been discovered and are nevertheless produced in ultra-modern enterprise.
Learn more about electrolysis here:- https://brainly.com/question/276473
#SPJ4
How many grams of H20 can be produced with 6.3 moles of O2?
Answers
Answer:
For the reaction
2
H
2
+
O
2
→
2
H
2
O
, how many moles of water can be produced from 6.0 mol of oxygen?
Chemistry Mole Ratios
1 Answer
Stefan V.
Dec 5, 2015
12 moles H
2
O
Explanation:
Your tools of choice for stoichiometry problems will always be the mole ratios that exist between the chemical species that take part in the reaction.
As you know, the stoichiometric coefficients attributed to each compound in the balanced chemical equation can be thought of as moles of reactants needed or moles of products formed in the reaction.
In your case, the balanced chemical equation for this synthesis reaction looks like this
2
H
2(g]
+
O
2(g]
→
2
H
2
O
(l]]
Notice that the reaction requires
2
moles of hydrogen gas and
1
mole of oxygen gas to produce
2
moles of water.
This tells you that the reaction produces twice as many moles of water as you have moles of oxygen gas that take part in the reaction.
You know that your reaction uses
6.0
moles of oxygen. Assuming that hydrogen gas is not a limiting reagent, you can say that the reaction will produce
6.0
moles O
2
⋅
2
moles H
2
O
1
moles O
2
=
12 moles H
2
O
At what temperature would 2.10 moles of N2 gas have a pressure of 1.25 atm and in a 25.0 Ltank?
Answers
The temperature at which 2.10 moles of N₂ gas would have a pressure of 1.25 atm in a 25.0 L tank is approximately 181.25 Kelvin.
To find the temperature for 2.10 moles of N₂ gas with a pressure of 1.25 atm in a 25.0 L tank, we can use the Ideal Gas Law equation, which is:
PV = nRT
where P represents pressure (in atm), V is the volume (in L), n is the number of moles, R is the ideal gas constant (0.0821 L atm/mol K), and T is the temperature (in Kelvin).
We are given the values for P (1.25 atm), V (25.0 L), and n (2.10 moles). We can rearrange the equation to solve for T:
T = PV / (nR)
Plugging in the values, we get:
T = (1.25 atm)(25.0 L) / [(2.10 moles)(0.0821 L atm/mol K)]
T = 31.25 / 0.17241
T ≈ 181.25 K
Therefore, the temperature at which 2.10 moles of N₂ gas would have a pressure of 1.25 atm in a 25.0 L tank is approximately 181.25 Kelvin.
Learn more about Ideal Gas Law equation here:
https://brainly.com/question/4147359
#SPJ11
What is a model and why do scientist use them?
Answers
Answer: In science, a model is a representation of an idea, an object or even a process or a system that is used to describe and explain phenomena that cannot be experienced directly. Models are central to what scientists do, both in their research as well as when communicating their explanations.
(hope this helps you ^^)
An ice cube melting is a physical change b. An ice cube melting is a chemical change c. Paper burning is a physical change d. Paper burning is a chemical change e. Bubbles forming when soda pop is poured into a glass is a physical change f. Bubbles forming when soda pop is poured into a glass is a chemical change g. Hard-boiling an egg is a physical change h. Hard-boiling an egg is a chemical change
Answers
Answer:
An ice cube melting is a physical change because melting doesn't change the composition of the element. Paper burning is a chemical change because combustion occurs. Bubbles forming in a soda does involve chemical changes. Hard-boiling an egg is a chemical change because the egg's composition changed.
Question 101 Homework Unanswered Fill in the Blanks Type your answers in all of the blanks and submit X₁ X Ω· H₂106 + Cr-10 + Cr³+ For the previous redox reaction, enter the correct coefficient
Answers
The correct coefficient for the previous redox reaction X₁ X Ω· H₂106 + Cr-10 + Cr³+ is 6.
In the given redox reaction, the coefficient in front of Cr³+ is 6. This means that 6 moles of Cr³+ ions are involved in the reaction. The coefficient indicates the relative amount of each species involved in the reaction. In this case, the reaction involves the transfer of electrons between species, with Cr³+ being reduced to Cr²+.
By assigning a coefficient of 6 to Cr³+, it ensures that the number of electrons transferred and balanced on both sides of the reaction equation.
The coefficient of 6 indicates that for every 6 moles of Cr³+ ions participating in the reaction, there must be a corresponding number of moles for the other species involved.
It is important to balance the coefficients in a redox reaction to ensure that the reaction obeys the law of conservation of mass and charge.
The balanced coefficients help in determining the stoichiometry of the reaction, providing a clear understanding of the relative amounts of reactants and products involved.
Learn more about redox reaction here: https://brainly.com/question/28300253
#SPJ11
calculate the mass percent of hoch2ch2oh in a solution made by dissolving 3.2 g of hoch2ch2oh in 43.5g of water.
Answers
TheThe mass percent of HOCH2CH2OH is 6.85%
Mass of solute (ethylene glycol) = 3.2 g
Mass of solvent (water) = 43.5 g
Mass of solution = Mass of solute + Mass of solvent = 3.2 + 43.5 = 46.7 g.
Mass%= mass of solute×100/mass of solution
Mass%=3.2×100/43.5 = 6.85%
What is the relationship between solute and solvent?
A solute is a substance this is dissolved in a solution. The quantity of solvent in a fluid solution is extra than the amount of solute. The solvent is the fabric that commonly comes to a decision the solution’s bodily nation (stable, liquid or gasoline). A solution of liquid and water, for example, has water because the solvent and salt because the solute. Water is also known as the “prevalent solvent” because it can dissolve almost any material better than some other liquid.To know more about solute and solvent, click the link given below:
httpshttps://brainly.com/question/16122098
#SPJ4
james chadwick discovered this particle, which was the last of the subatomic particles to be characterized.
Answers
James Chadwick discovered the neutron, which was the last of the subatomic particles to be characterized.
James Chadwick bombarded alpha particles on the beryllium surface using radioactive decay o polonium as a source. He discovered penetrating particles with no charge. These particles were fast-moving with no positive or negative charge.
These radiations were made incident on paraffin wax. This experiment was very similar to Rutherford's experiment.
The mass of the neutron was almost equal to the mass of the proton but its penetrating power was higher than a proton. It can penetrate several inches of lead.
If you need to learn more about neutron, click here
https://brainly.com/question/15120322?referrer=searchResults
#SPJ4
1. Calculate the number of moles in each of the following masses:
a. 64.1 g of aluminum ans: 2.38 mol Al show work
Answers
Answer:
2.38 mol of Al
Explanation:
To find the number of moles in a substance, you simply divide by the atomic mass. In this case, the conversion table would look as follows:
\(\frac{64.1 g}{1}\) * \(\frac{1 mol}{26.98 g}\)
The gram units cancel, and you are left with the answer of about 2.38 moles of Aluminum.
Help me Urgently!
To study the role of water in showing acidic properties

Answers
Answer:
When solid oxalic acid dissolved in water, it dissociates , H+ - ions is formed, so acidic properties can be observed.
Explanation:
When solid oxalic acid dissolved in water, it dissociates , H+ - ions is formed, so acidic properties can be observed.
C2H2O4 ----> \(H^{+}\) + \(C_{2}HO_{4}^{-}\)
\(C_{2}HO_{4}^{-} ----> H^{+} + C_{2}O_{4}^{2-}\)
When oxalic acid is solid, there are no free moving H+ - ions , so acidic properties can not be observed.
Carbon-14 has a half-life of 5,730 years. If a sample contained 80 mg originally, how much is left after 17,190 years? Show your work for complete credit.
Answers
Answer: 10 mg
Explanation:
First, take 17,190 divided by 5730 = 3 half-life
We know that after each half-life, the sample will decrease by half
80 mg = 0 half-life
40 mg = 1 half- life
20 mg = 2 half-life
10 mg = 3 half-life