The tendency of an object to resist a change.
A. Inertia
B. Friction
C. Law of conservation of motivation
D. Force
Answers
Answer:
inertia is the tendency of an object to resist a change
Related Questions
The end result of ecological succession is called?
1.) Primary Ecosystem
2.) Secondary Ecosystem
3.) Climax Community
4.) none of these
Answers
Convert 3.5mol of CO2 to grams.
Answers
How many centimeters are in 21.5 km?
Answers
Answer: 2150000 cm
Explanation:
Hey there!
In order to find the answer you have to MULTIPLY the kilometers (km) [21.5] from 100,000 and then you should be able to find your answer in centimeters (cm)
21.5 km * 100,000 = ? cm
Simplify that equation:
cm = 2,150,000
Therefore, your answer should be: 2,150,000
Good luck on your assignment & enjoy your day!
~Amphitrite1040:)
In a first-order decomposition reaction, 50.0% of a compound decomposes in 13.0 min. What is the rate constant of the reaction?
Answers
The rate constant of the reaction is 0.0531 min-¹.For a first-order reaction, the rate of the reaction is proportional to the concentration of the reactant. The rate law for a first-order reaction is given by the equation:
Rate = k[A]
where:
Rate is the rate of the reaction
k is the rate constant
[A] is the concentration of the reactant
The integrated rate law for a first-order reaction is:
ln([A]t/[A]0) = -kt
where:
[A]t is the concentration of the reactant at time t
[A]0 is the initial concentration of the reactant
k is the rate constant
t is the time
In this problem, we are given that 50.0% of a compound decomposes in 13.0 min. This means that [A]t/[A]0 = 0.5, and t = 13.0 min. Substituting these values into the integrated rate law, we get:
ln(0.5) = -k(13.0 min)
Solving for k, we get:
k = -ln(0.5)/13.0 min
k = 0.0531 min-¹
Therefore, the rate constant of the reaction is 0.0531 min-¹
To learn more about rate constant here:
https://brainly.com/question/20305871
#SPJ11
A compound containing only c, h, and o, was extracted from the bark of the sassafras tree. the combustion of 72.3 mg produced 196 mg of co2 and 40.2 mg of h2o. the molar mass of the compound was 162 g/mol. determine its empirical and molecular formulas
Answers
The Empirical formula of the compound is C₅H₅O.
and molecular formula of the compound is C₁₀H₁₀O₂.
mass of C in the compound = (196g CO2)x (12.0 g C/44.0 g CO2) = 53.45g C
mass of H in compound = (40.2 g H2O) x (2.0 g H /18.0 g H2O) = 4.46 g H
mass of O in compound = 72.3 g total - 53.45 g C - 4.46 g H = 14.39 g O
now convert mass of each element to moles
53.45 g C x (1 mole C/12.0 g C) = 4.45 moles C
4.46 g H x (1 mole H/1.0 g H) = 4.46 moles H
14.39 O x (1 mole O/16.0 g O) = 0.899 moles O
Divide each by 0.899 to get C₅H₅O, which has a mass of 81 g
double that to get C₁₀H₁₀O₂ with mass of 162 g.
This is a "classic" example of a combustion analysis problem.
To know more about empirical formula visit the link:
https://brainly.com/question/14044066?referrer=searchResults
#SPJ9
classify which compounds will dissolve in water and which ones will not dissolve in water.
will dissolve in water : will not dissolve in water :
Answers
Compounds that are ionic or polar in nature tend to dissolve in water, while compounds that are nonpolar or have strong intermolecular forces are less likely to dissolve in water.
When determining if a compound will dissolve in water, it is important to consider the nature of the compound's chemical bonds and intermolecular forces. Ionic compounds, such as sodium chloride (NaCl), readily dissolve in water due to the attraction between the charged ions and the polar water molecules. The positive ends of water molecules (hydrogen atoms) are attracted to the negative ions, while the negative ends of water molecules (oxygen atoms) are attracted to the positive ions.
Similarly, polar compounds, such as ethanol (C2H5OH), also dissolve in water because they have a positive and a negative region, allowing them to interact with water molecules through hydrogen bonding and dipole-dipole interactions.
On the other hand, nonpolar compounds, such as hydrocarbons like hexane (C6H14), have little to no polarity and do not readily interact with polar water molecules. Therefore, they are insoluble or have very low solubility in water.
In summary, compounds that are ionic or polar generally dissolve in water due to the favorable interactions between their chemical properties and the polar nature of water molecules. Nonpolar compounds, on the other hand, tend to be insoluble in water because they lack the necessary polarity to interact with water molecules effectively.
To know more about intermolecular forces, visit:-
https://brainly.com/question/12243368
#SPJ11
How many moles are in 3.5 of “K”?
Answers
Explanation: i don't know how can i know the moles in element?
the rays produced in a cathode tube are
Answers
Answer:
Electrons
Explanation:
Cathode rays carry electronic currents through the tube. Electrons were first discovered as the constituents of cathode rays. J.J. Thomson used the cathode ray tube to determine that atoms had small negatively charged particles inside of them, which he called “electrons.”
The rays produced in a cathode tube are electrons which are present in the shells surrounding the nucleus of an atom.
What is an atom?
An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ6
The gaseous state of a substance is due to:
A) the strong bonds between particles
B) the attractions between electrostatically charged particles
C) the high inertia of the small particles
D) the high inertia of the large particles
E) the high-speed motion of particles
Answers
Answer:
A the strong bonds between particles.
Explanation:
this is because in solids we say that they have a weak boling point due to the strong bond that makes it hard to melt
Oleic acid's melting point is 14°C and it's boiling point is 360°С. What phase is this substance
at room temperature 25°C?*
A. Solid
B. Liquid
C. Gas
D. Impossible to tell
Answers
Calculate the mass of acetone, C3H6O, that vaporizes as a result of absorbing 500 KJ of energy. The molar enthalpy of vaporization of acetone is 31.3 Kj/mol
Answers
The organic molecule acetone has the chemical formula (CH3)2CO. The smallest and most basic ketone. It is a colorless, incredibly combustible liquid with a distinctively strong smell. Because it is miscible with water, acetone is used as a significant organic solvent in industry, the household, and laboratories.
Calculate the mass of acetone, C3H6O, that vaporizes as a result of absorbing 500 KJ of energy.
Knowing that;
acetone weighs 31.5 g.
Acetone's molar mass is 58.08 g/mol.
Acetone has a heat of vaporization of 31.3 kJ/mol.
Count of moles = mass / molar mass
Acetone molecular weight = 31.5 / 580.8
Acetone has a molecular weight of 0.5424 moles.
By dividing the number of moles of acetone by the heat of vaporization of acetone, one may calculate the amount of heat necessary to evaporate 31.5 g of acetone;
Hence;
31.3 g of acetone must be heated to vaporization temperature of 31.0 kJ/mol, or 0.5424 mole.
16.8144 kJ of heat are needed to evaporate 31.3 g of acetone.
≅ 16.81 kJ
To know more about acetone, click on the link below:
https://brainly.com/question/15008900
#SPJ9
Some common substances and their chemical formulas are listed in the chart.
Which of these substances are elements?
Answers
Answer:
We heart you don't have all the information you just got some common substances and their chemical formulas are listed in the chart? That we don't have the chart to see which of the substances are elements
Explanation:
sorry about that if you can take a picture of it and repost it I am sure I could answer it for you
Based on your answer in part F, what must happen to the sodium acetate for it to dissolve in the water again? Safely test your method using only those materials available in the equipment list. Does the sodium acetate dissolve again with your chosen method?
Answers
When sodium acetate is dissolved in water, it is hydrolyzed into ions.
What happen when sodium acetate dissolved in water?When a salt of weak acid or base is dissolved in water, this salt hydrolysis in it. Water ionises into hydroxide and hydrogen due to addition of sodium acetate. In water, sodium acetate splits into sodium and acetate ions.
So we can conclude that when sodium acetate is dissolved in water, it is hydrolyzed into ions.
Learn more about dissolve here: https://brainly.com/question/12983022
A student is trying to identify an unknown metal X. When he puts it in copper sulphate there is a reaction and red brown pieces of copper fall to the bottom of the test tube. But when he puts metal X into magnesium chloride nothing happens
A) Give two identity of metal X.
B) Out of these two which one is metal X ?
Answers
The unknown metal X is iron metal as it reacts with copper sulfate solution but does not react with magnesium chloride.
What is displacement reaction?Some metals are very reactive while other metals are less reactive or unreactive. When a more reactive metal is added to the solution of a less reactive metal, then the more reactive displaces the less reactive metal from its solution is known as a displacement reaction.
The general form of a single displacement reaction can be represented as:
\(A + BC \longrightarrow B + AC\)
When iron is placed in copper sulfate (CuSO₄) solution then the blue color of the copper sulfate solution turns a red-brown coating of copper metal deposited on the iron.
\(CuSO_4 (aq)+ Fe (s)\longrightarrow FeSO_4 (aq) +Cu(s)\)
Iron lies above the electrochemical series and is more reactive than copper. So it reacts with copper sulfate but does not give any reaction with magnesium chloride.
Learn more about displacement reaction, here:
https://brainly.com/question/3172917
#SPJ1
A fire that is burning wood will release water vapor and carbon dioxide
true or false?
Answers
Answer:
true.
Explanation:
sodium atoms emit light with a wavelength of 330 nm when an electron moves from a 4p orbital to a 3s orbital. what is the energy difference between the orbitals in kj/mol
Answers
The energy difference between the orbitals can be calculated from bohr's model.
According to the bohr's model, the electrons are in continuous motion round the nucleus closed orbits of definite energy level. as the distance of the cell increases from the nucleus ,energy level of cell increases..as long as electron occupy a definite energy level, it doesn't radiate out energy. The emission of energy occurs only when electron jumps from one level to other.
E= h c/ λ
Sodium atom emit light with a wavelength of 330nm when an electron moves from a 4p orbital to a 3s orbital.
λ=330nm
h=6.625 .10-34 (Planck's constant)
c= 3.0 .108m/s-1
putting the values in the expression of Energy we get the energy difference.
To learn more about Bohr's model please visit:
https://brainly.com/question/18002213
$SPJ4
the gas-phase decomposition of s02cl2, s02cl2(g) ----> s02(g) cl2(g), is first order in s02cl2. at 600 k the half-life for this process is 2.3 x 105 s. what is the rate constant at this temperature? (b) at 320 oc the rate constant is 2.2 x 10--s s-1. what is the half-life at this temperature?
Answers
The gas-phase decomposition of SO2Cl2 is a first-order reaction, and the rate of the reaction is given by the equation: rate = k[SO2Cl2]
The half-life of a first-order reaction is given by the equation: T1/2 = 0.693/k (a) At 600 K, the half-life for the process is 2.3 x 105 s. To find the rate constant at this temperature, we can use the half-life equation to solve for k: T1/2 = 0.693/k ;2.3 x 105 s = 0.693/k ;k = 0.693 / 2.3 x 105 s = 3 x 10^-5 s-1 , (b) At 320 degree celcius , the rate constant is 2.2 x 10^-5 s^-1. To find the half-life at this temperature, we can use the half-life equation and substitute in the given rate constant: T1/2 = 0.693/k;T1/2 = 0.693 / 2.2 x 10^-5 s-1 ;T1/2 = 3.15 x 10^4 s Therefore, the half-life of the reaction at 320 degree celcius is 3.15 x 10^4 seconds.
Learn more about rate constant here:
https://brainly.com/question/20305922
#SPJ4
If 136 g of BaCl₂ (FW 208.23 g/mol) is added to a solution containing 95.0 g of Na₃PO₄ (FW 163.94 g/mol), what mass, in g, of Ba₃(PO₄)₂ (FW 601.92 g/mol) precipitate will be formed? The balanced equation is
3 BaCl₂(aq) + 2 Na₃PO₄(aq) → Ba₃(PO₄)₂(s) + 6 NaCl(aq)
Answers
Answer:
131g of Ba₃(PO₄)₂(s) precipitate will be formed
rate as brainliest

Answer:
131
Explanation:
What is the percent of O in
CO2?
Answers
Answer:
72.7% of oxygen
Explanation:
someone help me please....
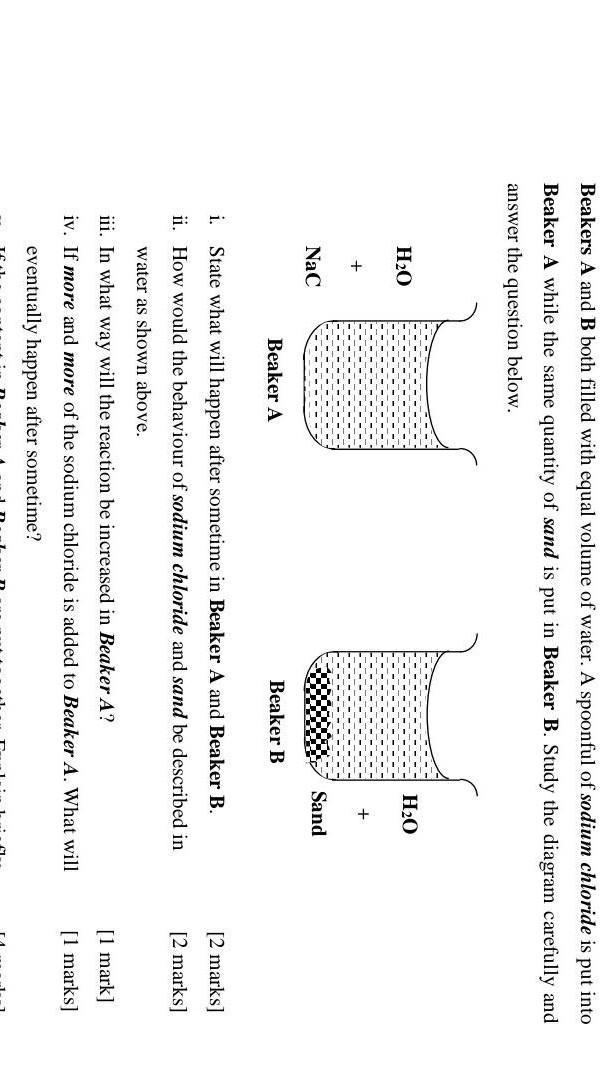
Answers
Answer:
i- In beaker A, sodium chloride will dissolve with water.
and in beaker B, there will be no reaction.
ii- sodium chloride is soluble in water, while sand is insoluble.
iii- The reaction can be increased by adding more sodium chloride to the beaker.
iv- The sodium chloride will no longer dissolve in the water.
select all correct answers! the primary cause of recent global warming is believed to be . group of answer choices chemical reactions in the atmosphere that are driven by solar heat increased burning of fossil fuels increased levels of atmospheric pollution depletion of the rain forests
Answers
The primary cause of recent global warming is believed to be increased burning of fossil fuels.
Fossil fuels such as coal, oil, and natural gas are used to produce energy for transportation, electricity, and heating. When these fuels are burned, they release carbon dioxide and other greenhouse gases into the atmosphere.Chemical reactions in the atmosphere that are driven by solar heat and increased levels of atmospheric pollution can also contribute to global warming, but they are not considered the primary cause.
Depletion of the rainforests can also have a negative impact on the climate, as they absorb carbon dioxide and release oxygen. The consequences of global warming are numerous and severe. They include rising sea levels, more frequent and intense natural disasters. It is essential that we take action to address this problem by reducing our dependence on fossil fuels and investing in renewable energy sources such as solar and wind power.
Know more about global warming here:
https://brainly.com/question/3553382
#SPJ11
The hybridizations of nitrogen in NF3 and NH3 are __________ and __________, respectively. A) sp2, sp2 B) sp3, sp3 C) sp, sp3 D) sp2, sp3 E) sp3, sp
Answers
The nitrogen hybridizations in NF3 and NH3 are, respectively, sp3 and sp3.
Describe hybridizations.When two atomic orbitals combine, a new degenerate hybrid orbital with the same energy levels is created. This phenomenon is known as hybridization. Compared to non-hybridized orbitals, hybridization increases bond formation stability. We can forecast a molecule's form thanks to its hybridization.Hybridization contributes to the explanation of molecular structure because the angles between bonds are essentially equivalent to the angles between hybrid orbitals.The nitrogen is sp3 hybridised, and as a result, it has four sp3 hybrid orbitals. Two of the sp3 hybridised orbitals overlap with hydrogen-derived s orbitals to create the two N-H sigma bonds. One of the sp3 hybridised orbitals overlaps with a carbon sp3 hybridised orbital to produce the C-N sigma bond.To learn more about hybridization visit:
https://brainly.com/question/22765530
#SPJ4
A student is investigating the motion of a pendulum. The student holds the bob in Position A and then releases it. Which statement is correct about the energy of the bob? Pendulum Fed point 2 O The potential energy of the bob is greatest in Position C Kinetic energy changes to potential energy as the bob moves from Position D to Position E The kinetic energy of the bob is greatest in Position A Potential energy changes to kinetic energy as the bob moves from Position C to Position D
Answers
The correct statement about the energy of the bob in the given scenario is: The potential energy of the bob is greatest in Position C.
In a pendulum, the bob's energy undergoes continuous transformation between kinetic energy (associated with motion) and potential energy (associated with position or height). As the bob moves, its energy changes between these two forms.
When the bob is at Position A, it is at its highest point in the swing, and its velocity is zero. At this point, the bob has maximum potential energy because it is at its maximum height from the reference point (usually the lowest point in the swing). Potential energy is directly related to the height or position above the reference point.
As the bob starts to move downward from Position A, its potential energy decreases, and its kinetic energy increases. At Position C, the bob reaches its lowest point in the swing, where its potential energy is at its minimum because it is closest to the reference point. At this point, the bob has converted all its potential energy into kinetic energy, as its velocity is at its maximum.
From Position C to Position D, as the bob starts moving upward, its kinetic energy decreases, and its potential energy increases again. The bob is gaining height, and its potential energy is being converted back into kinetic energy. This conversion continues until the bob reaches Position E, where it momentarily stops before starting its descent back to Position A.
In summary, the potential energy of the bob is greatest at Position C, while the kinetic energy is greatest at Position A. The bob's energy continuously changes between potential and kinetic as it swings back and forth.
To know more about potential energy, please click on:
https://brainly.com/question/24284560
#SPJ11
The pH of 0.010 M aniline(aq) is 8.32. What is the percentage aniline protonated? I know its .021% but i need to know how to get there.
Answers
The percentage of aniline protonated in a 0.010 M solution with a pH of 8.32 is approximately 0.021%.
Aniline (C6H5NH2) is a weak base that can be represented by the equation
C6H5NH2 + H2O ⇌ C6H5NH3+ + OH-.
The equilibrium constant expression for this reaction is
Kb = [C6H5NH3+][OH-]/[C6H5NH2].
At equilibrium, the concentration of aniline that has reacted to form the protonated form is [C6H5NH3+], and the concentration of aniline that has not reacted is [C6H5NH2].
Using the relationship between pH and [OH-] (pH = 14 - pOH), we can calculate that [OH-] = 10^(-5.68) M.
Using the equilibrium constant expression and the known concentration of aniline, we can solve for [C6H5NH3+]/[C6H5NH2], which is approximately 0.000213. This value multiplied by 100 gives the percentage of aniline that is protonated, which is approximately 0.021%.
For more questions like Aniline click the link below:
https://brainly.com/question/31081529
#SPJ11
A sample of radon gas has a volume of 1.53 L, a pressure of 1.15 atm, and a temperature of 305 K. What is the new pressure if the new volume is 1.78 and the new temperature is 325 K
Answers
Answer: 0.813 L
Explanation:

Ideal gas law is valid only for ideal gas not for vanderwaal gas. Combined Boyle's and Charles' gas law is used here. Therefore the new pressure of helium gas is 5.63 atm.
What is ideal gas equation?Ideal gas equation is the mathematical expression that relates pressure volume and temperature.
Mathematically the relation between Pressure, volume and temperature can be given as
PV=nRT
where,
P = pressure of radon gas
V= volume of radon gas
n =number of moles of radon gas
T =temperature of radon gas
R = Gas constant = 0.0821 L.atm/K.mol
Combining Boyle's and Charles' gas law
P₁V₁/T₁ = P₂V₂/T₂
{ (1.15 atm) (1.53 L)} ÷305 K ={ (P2) ( 1.78)} ÷325 K
P2 = 7.55÷1.34 = 5.63 atm
Therefore the new pressure of radon gas is 5.63 atm when the temperature is changed to 325K and volume is decreased to 1.78liter.
To learn more about ideal gas equation, here:
https://brainly.com/question/14826347
#SPJ5
Check the rate of evaporation in a wide mouthed container and narrow mouthed container. Note your observation during the peak of summer & the start of monsoon.
Answers
Answer:
Because of the summer season
Explanation:
The water in the dish which is uncovered will evaporate faster as compared to the one which is
covered. This is because the air above the uncovered dish has less water vapour over it as
compared to the one which is covered. The covered dish has good amount of moisture over it as
the closed lid does not allow the moisture to come in direct contact with air.
The shape of the methane molecule(ch4) is called
Answers
Answer:
Tetrahedral
Explanation:
 if your commute is 20 miles and you drive an average speed of 60 km/h how many minutes will it take you to get to work
Answers
Answer:
33 minutes
Explanation:
60 km/h = 37.28 mi/h
20/37.28 = 0.5364 h
0.5364 * 60 min = 32.2 minutes
Step 2: measure the area of the top of the syringe
Answers
I am unsure if this is correct, but this might be the whole section:
The top of the syringe is a circle. You need to compute its area for use in later computations of pressure values. Start by using a ruler to measure the diameter. Estimate to the nearest 0.01 cm. Answer: 3.60 cmDivide by two to find the radius. Maintain significant figures. Answer: 1.80 cmSubstitute the radius into the formula A = πr² to find the area of the top of the syringe. Maintain significant figures. Answer: 10.2 cm²A rectangular solid of unknown density is 5 meters long, 2 meters high and 4 meters wide. The mass of this solid is 300 grams. Given this information for this homogeneous (alike throughout) material, calculate its density.
Answers
Answer:
The density of the material is 7.5 g/m^3
Explanation:
The volume of a rectangle is:
Volume = Length*Height*Width
Replacing with data:
Volume = 5 m * 2 m * 4 m = 40 m^3
Density is computed as follows:
Density = Mass/Volume
Replacing with data:
Density = 300 g/40 m^3
Density = 7.5 g/m^3
(Answer creds: jbiain)