What is the density of a rectangle solid with dimensions
of 15 cm x 25 cm x 5 cm and has a mass of 3000 g?
Answers
Answer:
5625000gm/cm^3
Explanation:
volume=w*h*l=25*15*5=1875cm^3
density=mass*volume
=1875*3000
=5625000gm/cm^3
Related Questions
between vertebrae
2-The backbone contains.......
c. joints
b. tendons
a. cartilages
Answers
1. You may be using medium for shoot regeneration from leaf explants of a plant in Expt-5. The plant media may contain the plant growth regulators (hoones) BA and NAA. The molecular weight of BK is 72 A : and NAA is 186. The media is pH to 5.8. (a) Before making the plant media, you found the pH to be 3.6. What would you add quiekly to get it to a pH of 5.8 (give a specific name of the solution)? Why? (1 pt) (b) How much BA will be weighed fot a 1M solution? (Y po) (c) Convert your answer from (b) to mg/ml. (Y/ pt) (d) Convert your answer from (c) to mg 1 . (1 pt) (e) How much BA will be weighed for a 5mM solution? (1/4pt) (f) Convert your answer from (c) to mg/ml. ( /4pt ) (g) Convert your answer from (f) to mg/L. (H/ pt) (h) Your stock solution of BA is 5mM and your working solution is 0.2mg/.. What volume of the stoc be added to 250ml of medium? [Hint: fook at the previous answers Keep to 4 decimal pts.) (3 pts Convert your answer from (h) to μI, and which pipettor will you use to aliquot the B. A? (1 pt)
Answers
(a) To get the pH of the media to 5.8, you would add NaOH solution. NaOH is used as a basic solution, and when it is added to a solution, it will increase the pH of the solution.
(b) The molecular weight of BA is 225.3. To prepare a 1M solution, you would have to weigh out 225.3 grams of BA.(c) To convert a 1M solution of BA to mg/mL, you can use the following equation: 1 mole = molecular weight in grams; 1000 millimoles = 1 mole. So, 1 M = 1000 mg/mL. Therefore, a 1M solution of BA is equivalent to 1000 mg/mL .(d) To convert a concentration of 1000 mg/mL .
Therefore, to calculate the weight required for a 5 mM solution, use the following formula :Mass of BA = molarity × volume × molecular weight= 5 × 0.001 × 225.3= 1.1265 grams(f) To convert a concentration of 5 mM to mg/mL, we use the following formula: Concentration (mg/mL) = (Concentration (mM) × Molecular weight) / 1000= (5 × 225.3) / 1000= 1.1265 mg/mL(g)
To convert a concentration of 1.1265 mg/mL to mg/L, we multiply by 1000, so 1.1265 mg/mL = 1126.5 mg/L.(h) Given that the stock solution of BA is 5 mM and the working solution is 0.2 mg/mL.
To know more about increase visit:
brainly.com/question/19383315
#SPJ11
A sample of gas has an initial volume of 12.6 L at a pressure of 1.31 atm .
Part A If the sample is compressed to a volume of 10.7 L , what is its pressure?
Answers
When the gas is compressed to a volume of 10.7 L, its pressure is approximately 1.538 atm.
To determine the final pressure of the gas when it is compressed to a volume of 10.7 L, we can use Boyle's Law. Boyle's Law states that at a constant temperature, the pressure and volume of a gas are inversely proportional.
The formula for Boyle's Law is:
P1 * V1 = P2 * V2
Where P1 and V1 represent the initial pressure and volume, and P2 and V2 represent the final pressure and volume.
Given:
Initial volume (V1) = 12.6 L
Initial pressure (P1) = 1.31 atm
Final volume (V2) = 10.7 L (compressed volume)
We need to find the final pressure (P2).
Using Boyle's Law, we can rearrange the formula to solve for P2:
P2 = (P1 * V1) / V2
Substituting the given values:
P2 = (1.31 atm * 12.6 L) / 10.7 L
Calculating this expression:
P2 ≈ 1.538 atm
Therefore, when the gas is compressed to a volume of 10.7 L, its pressure is approximately 1.538 atm.
To learn more about Boyle's Law visit;
https://brainly.com/question/21184611
#SPJ11
In which direction does the moving force of air flow?
from areas of high pressure to areas of low pressure
from high elevations to low elevations
from east to west
from warm temperatures to cold temperatures
I will give brainliest
Answers
Answer:
from area of high pressure to area of low pressure
Explanation:
this phenomenon occurs due to the heating of Earth's surface by the sun which is quite uneven hence, causing the air flow from high pressure to a significantly lower pressure area.
A 2 cation of a certain transition metal has seven electrons in its outermost d subshell. Which transition metal could this be
Answers
The transition metal with a 2⁺ cation and seven electrons in its outermost d subshell could be either manganese (Mn) or technetium (Tc).
Transition metals have partially filled d subshells, which can form cations with different charges by losing electrons from the outermost shell. A 2⁺ cation indicates that the transition metal has lost two electrons, leaving behind the outermost d subshell with a specific number of electrons. Manganese (Mn) has an electron configuration of [Ar] 3d⁵ 4s², which means it has five electrons in its outermost d subshell.
If it loses two electrons to form a 2⁺ cation, it would have seven electrons in its outermost d subshell, as described in the question. Technetium (Tc) has an electron configuration of [Kr] 4d⁵ 5s², which means it also has five electrons in its outermost d subshell. If it loses two electrons to form a 2⁺ cation, it would have seven electrons in its outermost d subshell, similar to manganese.
To know more about the Transition metal, here
https://brainly.com/question/24146595
#SPJ1
Need help- dont know which category to put it in

Answers
Answer:
For reactants : Water , Carbon Dioxide , Sunlight
But for products : Oxygen , Glucose
Explanation:
A 56.88 g sample of a substance is initially at 20.4 °C. After absorbing 1295 J of heat, the temperature of the substance is 130.7 °C. What is the specific heat ( ) of the substance?
Answers
The specific heat of the substance is 0.20641 J/ g °C.
Given,
Q = 1295 J
Mass (m) = 56.88 g
ΔT = Final temperature - Initial temperature
ΔT = 130.7 °C - 20.4 °C
ΔT = 110.3 °C
We have to find the specific heat of the substance.
Q = m c ΔT
Where Q = amount of heat absorbed or released,
m = mass; c = specific heat
ΔT = change in temperature
Or, c = Q / m ΔT
c = 1295 / 56.88 × 110.3 °C
Or, c = 0.20641 J/ g °C
Therefore, the substance's specific heat is 0.20641 J/ g °C.
To learn more about specific heat, visit: https://brainly.com/question/21041726
#SPJ1
When will the forces of gravity between two objects be the GREATEST?
Answers
Hello There! My Name is Neko And I'll be helping you today!
Question:When will the forces of gravity between two objects be the GREATEST?
Also, you posted your question Under Chemistry And this question is physics Please remember to post under the correct subject
Answer:
the forces of gravity between two objects would be greatest if the distance between them is very small. They would be very close to each other.
Explanation:
According to Newton's gravitational law, any particle of matter in the universe attracts any other with a force varying directly as the product of their masses and inversely as the square of the distance between them. The formula is expressed as
F = Gm1m2/R^2
Where
F is the gravitational force
G is the gravitaional constant
m1 and m2 are the masses of the bodies
R is the distance between them
Looking at the formula, if we increase R, F would reduce. If we decrease R, F will increase. Thus, the forces of gravity between two objects would be greatest if the distance between them is very small. They would be very close to each other.
I hope This Helps!If you have any Doubts Please Tell meHave An Amazing Monday!
the volume of the granite as determined by water displacement is 9.35 ml. What iks the volume of the granite in cm3
Answers
The volume of the granite was determined by water displacement and is found to be 9.35 ml. The volume of granite in cm³ is 9.35 cm³
We know that, 1 ml = 1 cm³
We need to convert the volume from ml to cm³
The volume of the granite that was determined by water displacement is observed to be 9.35 ml.
After the conversion of volume of granite from ml to cm³, its volume in cm³ would be
1 ml = 1 cm³
9.35 ml = 9.35 cm³
Therefore, the volume of the granite that was determined by water displacement is 9.35 cm³
To know more about Volume
https://brainly.com/question/10051198
#SPJ1
Which element is a metal?
A. Ba
B. I
C. O
D. S
Answers
Answer:
A
Explanation:
hope this helps :)
Please answer the following question using the data below: H2O vapor content: 13 grams H2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10 ∘
C 52 grams at 30 ∘
C What is the dew point for the conditions listed above? LCL 3π5 25C Relative Humidity =100%
Answers
Given data:H2O vapor content: 13 gramsH2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10∘C52 grams at 30∘CFormula used to find the dew point:$$\dfrac{13}{52}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$\frac{1}{4}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$
Where A is the constantDew Point:It is the temperature at which air becomes saturated with water vapor when the temperature drops to a point where dew, frost or ice forms. To solve this question, substitute the given data into the formula.$$13/52=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$13(17.27-A)=3\pi A(ln100)$$By simplifying the above expression, we get$$A^2-17.27A+64.78=0$$Using the quadratic formula, we get$$A=9.9,7.4$$
The dew point is 7.4 since it is less than 10°C.More than 100:The term "More than 100" has not been used in the question provided.
To know more about temperature visit:
https://brainly.com/question/7510619
#SPJ11
The cultures of prehistoric humans are known mostly through the excavation of stone tools and other relatively imperishable artifacts. The early tool making traditions are often referred to as being paleolithic (literally "Old Stone Age). The Oldowan and Acheulian tool traditions of the first humans were the simplest applied research basic research Scientihe thought O philosophies technologies
Answers
The cultures of prehistoric humans are primarily known through the excavation of stone tools and other durable artifacts, such as the Oldowan and Acheulian tool traditions.
Stone tools and imperishable artifacts serve as key archaeological evidence for understanding prehistoric cultures. Through meticulous excavation and analysis, archaeologists have been able to piece together the lifestyles, technological advancements, and social behaviors of early human societies. The term "paleolithic" refers to the Old Stone Age, a time when humans relied on stone tools as their primary implements.
The Oldowan tool tradition is considered the earliest stone tool industry, dating back around 2.6 million years ago. It is characterized by simple tools, such as choppers and scrapers, which were crafted by flaking off pieces from larger stones. These tools were primarily used for basic activities like butchering and processing animal carcasses.
Later, the Acheulian tool tradition emerged around 1.76 million years ago, representing an advancement in stone tool technology. Acheulian tools, such as handaxes and cleavers, were more refined and standardized, showcasing an increased level of sophistication in tool-making techniques. These tools served a wide range of purposes, including hunting, woodworking, and shaping raw materials.
By studying the Oldowan and Acheulian tool traditions, researchers gain valuable insights into the cognitive abilities, cultural development, and technological progress of early humans. The examination of these artifacts provides evidence of their adaptability, problem-solving skills, and the gradual refinement of their tool-making techniques over time.
Learn more about prehistoric humans
brainly.com/question/28301954
#SPJ11
In this vLab you used a complex machine to launch a projectile with the ultimate goal of hitting a target. Assume you built a really big machine that could launch the projectile a “significant” distance; for instance, several hundred miles. Write a brief essay discussing the issues that would need to be accounted for with a projectile with that type of range. Be sure to include how those issues affect the range of the projectile.
Answers
Launching a projectile over a significant distance, such as several hundred miles, presents a range of complex challenges that must be carefully addressed. The success of achieving such a long range relies on accounting for various factors that influence the projectile's trajectory, including aerodynamics, atmospheric conditions, Earth's curvature, and external forces.
Air resistance can gradually decrease the projectile's speed, and the influence of wind could lead to the projectile drifting off the target. The size and shape of the projectile must be taken into consideration because these attributes can have a significant impact on the drag coefficient, which is a key factor in projectile performance. The larger the projectile's size, the more air resistance it will experience, lowering its range. The projectile's shape may cause the air to circulate over it, decreasing air resistance, which may result in a greater range. Finally, the materials used in the projectile's construction must be able to withstand the forces and heat generated when it is launched, particularly if it travels a long distance. The projectile must also be aerodynamic in order to be able to travel a long distance with ease.Thus, it can be concluded that the range of the projectile can be affected by factors such as air resistance, wind, size, shape, material, and aerodynamics.For such more questions on projectile
https://brainly.com/question/23827445
#SPJ8
If the universe is now expanding, why must it once have been a point?.
Answers
If the universe is expanding, why should it have been a point? The expanding universe, on the other hand, does not imply that the universe was once a point. The universe did not begin as a tiny speck that exploded into existence during the Big Bang.
That explanation is just a popular and incorrect portrayal of the event.The Big Bang is, in fact, a process in which the universe expands and cools over time. It's a process that takes place over time. In the very first instants of the Big Bang, the universe was hot and dense, but not a point. In fact, physicists believe that in the very early universe, the universe underwent a rapid process of expansion, known as inflation, which caused it to expand at a rate much faster than the speed of light. This expansion took place over a period of time, causing the universe to cool and expand, forming the stars and galaxies we see today.
:It is often believed that the universe began as a tiny speck that exploded during the Big Bang. This is, in fact, a misinterpretation of the event. The Big Bang is a process in which the universe expands and cools over time. Inflation, a period of rapid expansion that occurred in the early universe, is thought to have caused the universe to expand much faster than the speed of light. The universe cooled and expanded over time, forming the stars and galaxies we see today. Scientists' current understanding of the universe is that it has been continuously expanding since the Big Bang, and that this expansion is accelerating, driven by a mysterious force called dark energy.
In conclusion, the expanding universe does not suggest that it was once a point; rather, it implies that the universe has been expanding and cooling since the Big Bang, forming the stars and galaxies we see today.
To know more about Big Bang visit:
brainly.com/question/30671260
#SPJ11
Which of the following is used to identify a mineral
Carat, Cleavage, Cut, Clarity
Answers
Answer:
The physical properties of minerals are determined by the atomic structure and crystal chemistry of the minerals. The most common physical properties are crystal form, color, hardness, cleavage, and specific gravity. One of the best ways to identify a mineral is by examining its crystal form (external shape).
Which technology is shown in the photograph?
A. Negative controls
B. Biostimulation reaction
C. Gel electrophoresis
D. Polymerase chain reaction
Answers
Answer:
C
Explanation:
Apex
Find solutions for your homework
science
chemistry
chemistry questions and answers
given the following set of data, determine the enthalpy of formation of the nacl(s) from elemental na(s) and cl2(g) given the following information. lattice energy for solid nacl(s):-742kj/mol enthalpy of sublimation for solid na(s)->na(g):108kj/mol first ionization energy for sodium:496kj/mol cl-cl bond dissociation energy:75.3kj/mol electron affinity for
This problem has been solved!
You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
See Answer
Question: Given The Following Set Of Data, Determine The Enthalpy Of Formation Of The NaCl(S) From Elemental Na(S) And Cl2(G) Given The Following Information. Lattice Energy For Solid NaCl(S):-742kJ/Mol Enthalpy Of Sublimation For Solid Na(S)->Na(G):108kJ/Mol First Ionization Energy For Sodium:496kJ/Mol Cl-Cl Bond Dissociation Energy:75.3kJ/Mol Electron Affinity For
Given the following set of data, determine the Enthalpy of formation of the NaCl(s) from elemental Na(s) and Cl2(g) given the following information.
Lattice Energy for solid NaCl(s):-742kJ/mol
Enthalpy of sublimation for solid Na(s)->Na(g):108kJ/mol
First ionization energy for sodium:496kJ/mol
Cl-Cl bond dissociation energy:75.3kJ/mol
Electron affinity for chlorine:-349kJ/mol
If possible please type your answer and explanation.
Answers
The enthalpy of formation of NaCl(s) from elemental Na(s) and Cl₂(g) is -411.65 kJ/mol.
The enthalpy of formation for NaCl(s) can be calculated using the given data as follows;
Na(s) → Na(g)
ΔH1 = +108 kJ/mol
1/2Cl₂(g) → Cl(g)
ΔH2 = +121 kJ/mol
Na(g) → Na+(g) + e-
ΔH3 = +496 kJ/mol
Cl(g) + e- → Cl-(g)
ΔH4 = -349 kJ/mol
Na+(g) + Cl-(g) → NaCl(s)
ΔH5 = -742 kJ/mol
The enthalpy of formation of NaCl(s) from elemental Na(s) and Cl₂(g) can be calculated using Hess’s law.
Therefore;
ΔH5 = ΔH1 + ΔH2 + ΔH3 + ΔH4
Now, substituting the given data into the above equation we get;
-742 kJ/mol = +108 kJ/mol + 1/2(+121 kJ/mol) + (+496 kJ/mol) + (-349 kJ/mol) + ΔH4
Therefore;
ΔH4 = -75.3 kJ/mol
Thus, the enthalpy of formation of NaCl(s) from elemental Na(s) and Cl₂(g) is given as;
ΔH5 = ΔH1 + ΔH2 + ΔH3 + ΔH4= 108 kJ/mol + 1/2(121 kJ/mol) + 496 kJ/mol - 349 kJ/mol - 75.3 kJ/mol
= -411.65 kJ/mol
Therefore, the enthalpy of formation of NaCl(s) from elemental Na(s) and Cl₂(g) is -411.65 kJ/mol.
To know more about Hess’s law, visit:
https://brainly.com/question/31508978
#SPJ11
how have humans effected climate change?
Answers
The anthropogenic activities such as the release of carbon dioxide has contributed to climate change.
What is climate change?Large amounts of carbon dioxide are released into the atmosphere via the combustion of fossil fuels like coal, oil, and natural gas for energy production, transportation, and industrial activities. A greenhouse gas called carbon dioxide traps heat in the atmosphere of the Earth, causing the greenhouse effect and global warming.
Large forested areas have been lost as a result of deforestation, which is mostly caused by logging, urbanization, and agricultural development. As part of their photosynthesis, trees serve as carbon sinks by absorbing carbon dioxide.
Learn more about climate change:https://brainly.com/question/31604908
#SPJ1
21.One calcium atom (Ca+2) will combine with which of the following atoms in a one-to-one ratio?Select one:a. Be+2b. O-2c. Cl-d. Na+
Answers
O2- Option B is correct
Explanations:
Calcium atom is a group 2 elements that gives out two electrons in order to form a stable configuration and a Ca2+ ion. These two electrons are transferred to an atom that required two electrons to complete its outer shell.
Since oxygen required 2 electron to attain its octet configuration, hence they can easily accepts the two electrons from calcium atoms to form CaO which is composed of Ca2+ and O2- ions in the ratio of one Ca2+ ion to one O2− ions.
A compound contains 40.0% C, 6.71% H, and 53.29% O by mass. The molecular weight of the compound is 60.05 amu. The molecular formula of this compound is 1. C2H4O2 2. C2H204 3. CH20 4. CHO2 C 5. C2H304
Answers
Correct answer is C2H4O2. The empirical formula of a compound represents the simplest ratio of the elements present in the compound. It shows the relative number of atoms of each element in the compound.
To determine the molecular formula of the compound, we need to calculate the empirical formula first. The empirical formula represents the simplest ratio of the elements present in the compound.
Given the mass percentages of carbon (C), hydrogen (H), and oxygen (O), we can assume a 100 g sample of the compound. This means we have:
Mass of C = 40.0 g
Mass of H = 6.71 g
Mass of O = 53.29 g
Next, we need to convert these masses into moles. We can use the molar mass of each element to convert from grams to moles:
Molar mass of C = 12.01 g/mol
Molar mass of H = 1.008 g/mol
Molar mass of O = 16.00 g/mol
Now, let's calculate the number of moles for each element:
Moles of C = Mass of C / Molar mass of C = 40.0 g / 12.01 g/mol ≈ 3.33 mol
Moles of H = Mass of H / Molar mass of H = 6.71 g / 1.008 g/mol ≈ 6.66 mol
Moles of O = Mass of O / Molar mass of O = 53.29 g / 16.00 g/mol ≈ 3.33 mol
The empirical formula is the simplest whole number ratio of the elements, so we divide each mole value by the smallest mole value (in this case, 3.33 mol):
Moles of C (simplified) = 3.33 mol / 3.33 mol = 1
Moles of H (simplified) = 6.66 mol / 3.33 mol ≈ 2
Moles of O (simplified) = 3.33 mol / 3.33 mol = 1
Based on these simplified mole ratios, the empirical formula of the compound is CH2O.
To find the molecular formula, we need to compare the empirical formula mass (sum of the atomic masses in the empirical formula) with the given molecular weight of 60.05 amu.
Empirical formula mass of CH2O:
= (1 * molar mass of C) + (2 * molar mass of H) + (1 * molar mass of O)
= (1 * 12.01 g/mol) + (2 * 1.008 g/mol) + (1 * 16.00 g/mol)
≈ 30.03 g/mol
To find the molecular formula, we divide the molecular weight by the empirical formula mass and round to the nearest whole number:
Molecular weight / Empirical formula mass = 60.05 amu / 30.03 g/mol ≈ 2
Therefore, the molecular formula of the compound is 2 times the empirical formula:
Molecular formula = 2 * CH2O = C2H4O2
The molecular formula of the compound is C2H4O2.
To know more about empirical formula, visit:
https://brainly.com/question/1439914
#SPJ11
a neutral atom is bombarded with sufficient energy that an electron is liberated. the result is called
Answers
Answer:
A cation.
Explanation:
When an electron is liberated, that means it is removed from the atom.
When an electron is lost, the atom is ionized, and becomes a cation.
It becomes a cation because it loses one negative charge, and therefore become positively charged.
James chadwick, like ernest rutherford, had to infer that neutrons were present in
atoms. he made inferences based on observations of the behavior of atoms during
experimental tests. using evidence from rutherford's gold foil experiment, defend the
following claim: scientific knowledge is based on observation and inference.
Answers
The claim that scientific knowledge is based on observation and inference is that science is always about empirical evidence , and when making observations , we can get some evidences and inference which serves a conclusion gotten on basis of evidence and reasoning.
What is scientific knowledge ?Science knowledge serves as the knowledge that is based and focus on empirical evidence.
It should be noted that in Science disciplines their is sharing of common rules of evidence which is needed in evaluation of explanations about natural systems.
Read more about scientific knowledge ;
brainly.com/question/25961563
#SPJ1
Imagine that you were to conduct an experiment by placing an aluminum wire into a solution of nickel chloride: 2 Al(s) + 3NiCl2(aq) What are the products of this reaction? A. AlCl3(aq) + Ni(s) B. 2 Al(s) + 3 Ni(s) + 3 Cl2(g)
D. No reaction occurs, so there are no products. E. 2A1C13(aq) + 3 Ni(s)
Answers
The products of the reaction between aluminum and nickel chloride are aluminum chloride (AlCl₃) in aqueous solution and solid nickel (Ni).
Option A is the correct answer: AlCl₃(aq) + Ni(s).
How can the products of the reaction between aluminum and nickel chloride be determined?The products of the reaction between aluminum (Al) and nickel chloride (NiCl2) can be determined by balancing the chemical equation and considering the reactivity of the elements involved. The balanced equation for the reaction is:
2 Al(s) + 3 NiCl2(aq) → 2 AlCl₃(aq) + 3 Ni(s)
In this reaction, aluminum (Al) is oxidized and loses electrons, while nickel (Ni) is reduced and gains electrons. The aluminum atoms from the solid react with the nickel chloride in the aqueous solution, resulting in the formation of aluminum chloride (AlCl₃) in aqueous form and solid nickel (Ni) as the products.
Aluminum chloride (AlCl₃) is an ionic compound consisting of Al₃+ cations and Cl- anions. It dissolves in water to form an aqueous solution. Nickel (Ni) is a transition metal that exists in a solid state.
The reaction does occur and follows a redox process, where one element is oxidized (Al) and another is reduced (Ni). This type of reaction is often referred to as a single replacement reaction because one element is replacing another in a compound.
It's important to note that the option "2 Al(s) + 3 Ni(s) + 3 Cl2(g)" (Option B) is not correct since it suggests the formation of chlorine gas (Cl2), which is not a product in this particular reaction.
By understanding the balanced equation and the nature of the reactants and products, we can conclude that the reaction between aluminum and nickel chloride results in the formation of aluminum chloride (AlCl₃) in aqueous form and solid nickel (Ni).
Therefore, option A is the correct answer: AlCl₃(aq) + Ni(s).
Learn more about nickel chloride
brainly.com/question/16751872
#SPJ11
3.0 mol Na reacts with 1.4 mol
F2 according to the equation below:
2Na+ F₂ → 2NaF
How many moles of NaF form
from 3.0 mol Na?
Answers
The number of moles of NaF that will be produced from 3moles of Na is 3 moles.
How to calculate number of moles?Stoichiometry is the study and calculation of quantitative (measurable) relationships of the reactants and products in chemical reactions (chemical equations).
According to this question, sodium reacts with fluorine as follows:
2Na+ F₂ → 2NaF
Based on the above equation, 2 moles of Na will produce 2 moles of NaF
This means that 3 moles of Na will produce 3 moles of NaF.
Learn more about stoichiometry at: https://brainly.com/question/9743981
#SPJ1
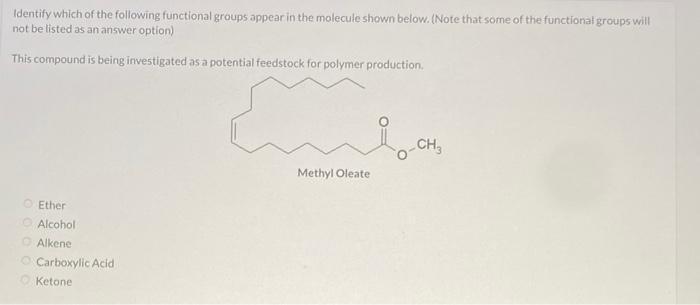
identify which of the following functional groups appear in the molecule shown below. (note that some of the functional groups will not be listed as an answer option)
Answers
The functional groups that we have in the compound are ketone and alkene. Options C and E
What is a functional group?
A functional group is a particular set of atoms in a molecule that are in charge of the molecule's distinctive chemical processes and characteristics. The behavior and functionality of a molecule in chemical processes are determined by a reactive component of the molecule.
The common atoms found in functional groups are carbon, hydrogen, oxygen, nitrogen, sulfur, and phosphorus. They can be recognized by their unique atom arrangement and bonding structure inside a molecule.
Learn more about functional group:https://brainly.com/question/1356508
#SPJ4
Why are some elements classified as metalloids
Answers
An element with properties halfway between those of metals and nonmetals is said to be a metalloid. Semimetals are another name for metaloids.
Why are certain elements referred to as metalloids?A metalloid is an element that is difficult to categorize as either a metal or a nonmetal because it has a majority of qualities that fall between those of metals and nonmetals or that are a combination of both.
How are metalloids recognized?Except for hydrogen, which is a nonmetal, the elements are arranged in a triangle with the metals to the left, the nonmetals to the right, and the metalloids to the immediate right of the triangle.
To know more about metalloid visit:-
https://brainly.com/question/2548493
#SPJ1
a random sample of 160 united airline flights found that 140 arrived on-time. expedia would like to set α = 0.05. which one of the following statements is true?
Answers
The statement that is true is that the percentage of on-time United Airlines flights is 87.5%. The test statistic is calculated using the formula:
\($$\frac{{\left( {Observed\;Value} \right) - \left( {Expected\;Value} \right)}}{SE}$$\)
Here, the observed value is 140 and the expected value is calculated as follows:
Expected Value = (Total Number of Flights) × (Percentage of On-Time Flights)/100
Expected Value = 160 × (140/160)
Expected Value = 140
Thus, the observed and expected values are the same. Hence, the value of the test statistic is zero, which means that we fail to reject the null hypothesis, i.e., we accept that the percentage of on-time United Airlines flights is 87.5%.
Therefore, the statement that is true is that the percentage of on-time United Airlines flights is 87.5%.
To know more about test statistic, refer
https://brainly.com/question/15110538
#SPJ11
The physical and chemical breakdown of rock exposed to wind, water, ice and living orgeninns is: Regolith Soil
This process breaks down rocks with less surface area to pieces with more surface area D
Answers
The physical and chemical breakdown of rock exposed to wind, water, ice, and living organisms results in the formation of regolith and soil. This process involves the fragmentation of rocks into smaller pieces, increasing their surface area, which facilitates further weathering and erosion.
The breakdown of rock through various physical and chemical processes is known as weathering. When rock is exposed to external forces such as wind, water, ice, and living organisms, it undergoes mechanical and chemical weathering.
Mechanical weathering includes processes like abrasion, where rocks are physically broken down into smaller fragments by wind, water, or ice. This fragmentation increases the surface area of the rocks.
Chemical weathering occurs when the minerals in the rock react with water, gases, or other substances present in the environment. Chemical reactions can alter the composition of the minerals, leading to their decomposition or dissolution.
This further contributes to the breakdown of rocks and the generation of regolith, which refers to the layer of loose, fragmented material that covers solid bedrock.
Over time, the accumulation of organic matter in the regolith, along with the weathered rock material, creates a fertile layer called soil. Soil provides a habitat for plants and supports their growth by supplying nutrients and water.
The continuous breakdown of rocks and the addition of organic matter contribute to the development of soil, which plays a crucial role in sustaining ecosystems and supporting agricultural activities.
Learn more about Chemical weathering here: https://brainly.com/question/29616569
#SPJ11
Which part of a chemical equation is the section that gets rearranged
Answers
Chemical reactions cause the bonds between the atoms in the reactants to rearrange to create new compounds , but no atoms vanish or are created.
Chemical equations are symbolic depictions of chemical reactions where the reactants and products are stated in terms of their respective chemical formulae.
A molecule changes into a different chemical species when light causes it to rearrange its structure, losing atoms in the process. The transformation of 7-dehydrocholesterol to vitamin D in the skin is one biologically significant photorearrangement event.
In a chemical reaction, reactants combine to generate products (new substances). The molecules' bonds break when energy is absorbed in the process, and they then reorganize to make new bonds.
To know about equation
https://brainly.com/question/28312423
#SPJ4
I will give you brainliest

Answers
Answer:
Plumbing System
Explanation:
Answer:
C plumbing system
Explanation:
Infrastructure is the basic structures needed for the operation of society