Answers
The enthalpy change for producing 1.00 L of acetic acid for the given reaction is -2.34 kJ.
In order to calculate the enthalpy change for producing 1.00 L of acetic acid (CH3CO2H), we first need to use the given reaction equation and also the density of acetic acid to determine the number of moles produced.
After that, we can use the molar enthalpy of reaction to further calculate the enthalpy change.
Firstly for this question, we need to convert the volume of acetic acid (1.00 L) to its mass using the density (1.044 g/mL):
mass = volume x density
= 1.00 L x 1.044 g/mL = 1.044 g
In next step, we need to convert the mass of acetic acid to moles:
moles = mass / molar mass
= 1.044 g / 60.05 g/mol = 0.01739 mol
Now finally, we can use the molar enthalpy of reaction to calculate the final answer of enthalpy change:
\(ΔH = (mol CH3CO2H) x (ΔHrxn / mol CH3CO2H)\) = 0.01739 mol x (-134.6 kJ/mol) = -2.34 kJ
Hence, the enthalpy change for producing 1.00 L of acetic acid by the given reaction is -2.34 kJ.
The negative sign in the answer indicates that the reaction is exothermic in nature, meaning that it releases heat to the surroundings.
To know more about enthalpy change refer to-
https://brainly.com/question/29556033
Related Questions
et D Q.No. 9 Convert 1-bromopropane to 2-bromopropane.[2]
Answers
1-bromopropane can be converted to 2-bromopropane through a substitution reaction with sodium iodide in acetone.
Conversion of 1-bromopropane to 2-bromopropaneOne possible method to convert 1-bromopropane to 2-bromopropane is through a substitution reaction with sodium iodide (NaI) in acetone. The following procedure can be followed.
Dissolve 1-bromopropane and sodium iodide in dry acetone in a round-bottom flask.Reflux the mixture for several hours with stirring and heating to about 50-60°C. This allows for the formation of an alkyl iodide intermediate.After cooling the reaction mixture, add water to it and extract the organic layer with a separating funnel.Dry the organic layer with anhydrous sodium sulfate (Na2SO4) and filter it to remove any solid impurities.Concentrate the organic layer by distillation under reduced pressure to obtain 2-bromopropane as a product.More on 1-bromopropane can be found here: https://brainly.com/question/31149600
#SPJ1
I’m not good at chemistry

Answers
Answer:
λ = 5.56 × 10⁻³ m
Explanation:
You have to use the formula c = λv to solve the problem. Review what you are given. You are given v and c, where v = 5.40 × 10¹⁰ Hz and c = 3.00 × 10⁸ m/s. The value c is for the speed of light and is something you need to memorize. You will use it often in physics and sometimes in chemistry.
Now that you figured out what you know, you can see that there is only one unknown, allowing you to solve.
c = λv
λ = c/v
λ = (3.00 × 10⁸ m/s)/(5.40 × 10¹⁰ Hz)
λ = 5.56 × 10⁻³ m
What determines how ocean currents move?
Answers
Answer: gravity wind and water density or surface currents
Explanation:
Hope this helped
What is the molar mass for dinitrogen trioxide
Answers
Answer
The molar mass of N₂O₃ = 76.0104 g/mol
Step-by-step explanation:
Using the atomic masses of elements from the periodic table; (N = 14.0067, O = 15.999).
Therefore, the molar mass of dinitrogen trioxide is calculated below.
N₂O₃ = 2(N) + 3(O)
N₂O₃ = 2(14.0067) + 3(15.999)
N₂O₃ = 28.0134 + 47.997
N₂O₃ =76.0104 g/mol
The molar mass of N₂O₃ = 76.0104 g/mol
The initial temperature of the water in a constant-pressure calorimeter is
14°C. A reaction takes place in the calorimeter, and the temperature rises
to 87°C. The calorimeter contains 254 g of water, which has a specific heat
of 4.18 J/(g.°C). Calculate the enthalpy change during this reaction. *
Answers
Answer: The enthalpy change during this reaction is 77505.56 J.
Explanation:
Given: \(T_{1} = 14^{o}C\), \(T_{2} = 87^{o}C\)
Mass = 254 g, Specific heat = \(4.18 J/g^{o}C\)
Formula used to calculate the enthalpy change is as follows.
\(q = m \times C \times (T_{2} - T_{1})\)
where,
q = enthalpy change
m = mass of substance
C = specific heat capacity
\(T_{1}\) = initial temperature
\(T_{2}\) = final temperature
Substitute the values into above formula as follows.
\(q = m \times C \times (T_{2} - T_{1})\\= 254 g \times 4.18 J/g^{o}C \times (87 - 14)^{o}C\\= 77505.56 J\)
Thus, we can conclude that the enthalpy change during this reaction is 77505.56 J.
The following Lewis diagram represents the valence electron configuration of a main-group element.
This element is in group
.
According to the octet rule, this element would be expected to form an ion with a charge of
.
If is in period 5, the ion formed has the same electron configuration as the noble gas
.
The symbol for the ion is
.
Answers
This element is in group 1.
According to the octet rule, this element would be expected to form an ion with a charge of +1.
If X is in period 5, the ion formed has the same electron configuration as the noble gas Krypton
The symbol for the ion is Rb⁺
What is electronic configuration?Electronic configuration refers to the arrangement of electrons in the orbitals of an atom or molecule, indicating the energy level of the electrons, the number of electrons in each energy level, and the number of electrons in each orbital.
Considering the given element:
It has one valence electron, hence it is in group 1. Group 1 elements form ions with a charge of +1.
Losing one electron will give the ion the same electron configuration as Kyrton since it is the noble gas in Period 4.
The element is rubidium and the ion is Rb⁺.
Learn more about electronic configuration at: https://brainly.com/question/26084288
#SPJ1

A gaseous element that is a poor conductor of electricity,
and does not react with other elements is classified as a

Answers
Answer:
Nonmetals are elements that generally cannot conduct electricity. They are the second largest class of elements after metals. Examples of nonmetals include hydrogen, carbon, chlorine, and helium. Properties of nonmetals include a relatively low boiling point, so many nonmetals are gases.
It would be considered a Nonmetal
Explanation:
What percentage of Americans believes that humans are causing global warming?
10
24
68
93
Answers
Answer:
49% say human activitiy contributes global warming.
Answer:
The answer is 68%
Explanation:
including me :)
Calculate the standard enthalpy change for the reaction
2A+B⇌2C+2D
Use the following data:
Substance ΔH∘f
( kJ/mol)
A -231
B -409
C 177
D -515
Express your answer to three significant figures and include the appropriate units.
Answers
The standard enthalpy change for the reaction 2A + B ⇌ 2C + 2D is 195 kJ/mol.
What is the enthalpy of a reaction?The enthalpy of a reaction is a measure of the heat changes that occur when reactants form a product in a given reaction.
The enthalpy change of a reaction can be calculated using the formula given below:
Enthalpy of reaction = sum of enthalpies of the products - sum of enthalpies of the reactants
Considering the given reaction: 2A + B ⇌ 2C + 2D
Enthalpy of reaction = (2 * C + 2 * D) - (2 * A + B)
Enthalpy of reaction = {[2 * 177 + 2 * (-515)] - [2 * (-231) + (-409)]}
Enthalpy of reaction = 195 kJ/mol
Learn more about enthalpy of reaction at: https://brainly.com/question/14291557
#SPJ1
Hope Sergil
Check the Science and Interpreting Graphs
A group of students buit electromagnets using, wre, nails, and a 6V lentern battery. They decided design an
one set of investigations Use the graph to complete the organizer
nvestigation to test factors that they could chance to make a stronger electromagnet. The follow dete is from
Number of Wire Wraps and Magnetic Strength
Paperclips Magnet Picks Up
Independent Variable
Dependent Variable
12
10
8
6
4
Possible Research Question
What trends do you notice in the
data?
Write a claim based on this data
What evidence from the chart or
graph supports your claim?
25
vat
50
Number of Coils Wrapped Around
000
75
Answers
Answer:
Independent Variable: Number of Coils Wrapped Around
Dependent Variable: Number of Paperclips Magnet Picks Up
Possible Research Question: How does the number of wire wraps affect the strength of an electromagnet?
Trends noticed in the data: As the number of wire wraps (coils) around the nail increases, the magnetic strength of the electromagnet also increases. There is a positive correlation between the number of wire wraps and the number of paper clips the magnet picks up.
Claim based on the data: The magnetic strength of an electromagnet increases as the number of wire wraps (coils) around the nail increases.
Evidence from the chart or graph supporting the claim: The graph shows that as the number of wire wraps increases from 4 to 12, the number of paper clips the magnet picks up also increases from 6 to 25. This suggests a positive correlation between the two variables.
The volume of a gas is 200 mL at 350.0 kPa pressure. What will the volume be when the pressure is reduced to 125.0 kPa, assuming the temperature remains constant.
Answers
The volume of the gas will be 560 mL when the pressure is reduced to 125.0 kPa, assuming the temperature remains constant.
What will be the volume of the gas when the pressure is reduced to 125.0 kPa?Boyle's law simply states that "the volume of any given quantity of gas is inversely proportional to its pressure as long as temperature remains constant.
Boyle's law is expressed as;
P₁V₁ = P₂V₂
Where P₁ is Initial Pressure, V₁ is Initial volume, P₂ is Final Pressure and V₂ is Final volume.
Substituting the given values, we get:
P₁ = 350.0 kPa (initial pressure)
V₁ = 200 mL (initial volume)
P₂ = 125.0 kPa (final pressure)
V₂ = ?
Solving for V₂, we get:
V₂ = ( P₁ × V₁ ) / P₂
V₂ = (350.0 kPa × 200 mL) / 125.0 kPa
V₂ = 560 mL
Therefore, the final volume of the gas is 560 mL.
Learn more about Boyle's law here: brainly.com/question/1437490
#SPJ1
Determine the molar concentration of acetic acid using the titration graph midpoint (10.25 = midpoint):
given:
concentration of NaOH = 0.60mol/L
volume of acetic acid = 25mL (or 0.025L)

Answers
The molar concentration of acetic acid using the titration graph midpoint. The molar concentration of acetic acid is 0.246 mol/L
The balanced chemical equation for the reaction between acetic acid and sodium hydroxide (NaOH) may be used to get the molar concentration of acetic acid (CH₃COOH):
CH₃COOH + NaOH → CH₃COONa + H2O
Concentration of NaOH (NaOH)) = 0.60 mol/L
Volume of acetic acid (CH₃COOH)) = 0.025 L
The halfway volume is 10.25 mL, or 0.01025 L, as indicated by the titration graph.
Multiply the NaOH concentration by the volume utilised at the halfway to determine the moles of NaOH used:
Moles of NaOH (n(NaOH)) = C(NaOH) × V(NaOH)
n(NaOH) = 0.60 mol/L × 0.01025 L
The reaction is 1:1, the moles of NaOH used are equal to the moles of acetic acid:
Moles of acetic acid (n(CH3COOH)) = n(NaOH)
Now we can calculate the molar concentration of acetic acid:
Molar concentration of acetic acid (C(CH3COOH)) = n(CH3COOH) / V(CH₃COOH)
C(CH₃COOH) = n(CH3COOH) / 0.025 L
Place the values:
C(CH₃COOH) = (0.60 mol/L × 0.01025 L) / 0.025 L
C(CH₃COOH) = 0.246 mol/L
Thus, the molar concentration of acetic acid is 0.246 mol/L.
Learn more about molar concentration, here:
https://brainly.com/question/15229198
#SPJ1
what is solubility? .
Answers
What is the limiting reagent when a 2.00 g sample of ammonia is mixed with 4.00 g of oxygen?
Answers
Answer:
Ammonia is limiting reactant
Amount of oxygen left = 0.035 mol
Explanation:
Masa of ammonia = 2.00 g
Mass of oxygen = 4.00 g
Which is limiting reactant = ?
Balance chemical equation:
4NH₃ + 3O₂ → 2N₂ + 6H₂O
Number of moles of ammonia:
Number of moles = mass/molar mass
Number of moles = 2.00 g/ 17 g/mol
Number of moles = 0.12 mol
Number of moles of oxygen:
Number of moles = mass/molar mass
Number of moles = 4.00 g/ 32 g/mol
Number of moles = 0.125 mol
Now we will compare the moles of ammonia and oxygen with water and nitrogen.
NH₃ : N₂
4 : 2
0.12 : 2/4×0.12 = 0.06
NH₃ : H₂O
4 : 6
0.12 : 6/4×0.12 = 0.18
O₂ : N₂
3 : 2
0.125 : 2/3×0.125 = 0.08
O₂ : H₂O
3 : 6
0.125 : 6/3×0.125 = 0.25
The number of moles of water and nitrogen formed by ammonia are less thus ammonia will be limiting reactant.
Amount of oxygen left:
NH₃ : O₂
4 : 3
0.12 : 3/4×0.12= 0.09
Amount of oxygen react = 0.09 mol
Amount of oxygen left = 0.125 - 0.09 = 0.035 mol
how many moles are in a 25.0 grams of carbon dioxide
Answers
answer:44.0095
Explanation:
sana makatulong
Answer:
44.0095
Explanation:
We assume you are converting between grams CO2 and mole. You can view more details on each measurement unit: molecular weight of CO2 or mol This compound is also known as Carbon Dioxide. The SI base unit for amount of substance is the mole.
Write a net ionic equation to show that triethylamine, (C2H5)3N, behaves as a Bronsted-Lowry base in water.
Answers
Answer:
\((C_2H_5)_3N~+~H_2O~->~(C_2H_5)_3NH^+~+~OH^-\)
Explanation:
For this question, we have to remember that definition of acid and base in the Bronsted-Lowry theory:
Acid
A substance with the ability to produce a hydronium ion (\(H^+\)).
\(HA~->~H^+~+~A^-\)
Base
A substance with the ability to accepts a hydronium ion (\(H^+\)).
\(B~+~H^+->BH^+\)
If we check the reaction mechanism (figure 1). We can see that the lone pair of electrons in the "N" atom will remove an "H" from the water molecule producing a positive charge in the nitrogen and a hydroxyl group (\(OH^-\)).
With all this in mind, the net ionic equation would be:
\((C_2H_5)_3N~+~H_2O~->~(C_2H_5)_3NH^+~+~OH^-\)
I hope it helps!

Give the number of significant figures in the following measurement 1278.50
Answers
Answer:
5 significant figures all figures are significant except for 0
Look at picture and answer will mark brainliest.
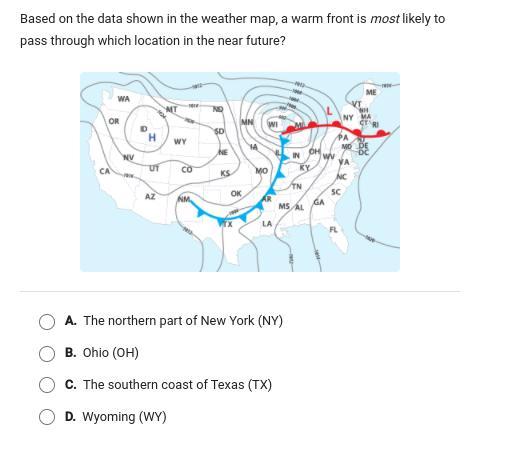
Answers
Answer:
A
Explanation:
Is a nonmetal a pure substance containing only one type of atom?
Your answer:
o No-nonmetals are compounds and compounds are a mixture of different types of atoms.
o Yes- nonmetals are compounds and compounds are pure substances containing only one type of atom.
O No-nonmetals are elements and elements are a mixture of different types of atoms.
o Yes-nonmetals are elements and elements are pure substances containing only one type of atom.
Clear answer
Answers
The reactant concentration in a zero-order reaction was 8.00×10−2 M
after 140 s and 4.00×10−2 M after 400 s
. What is the rate constant for this reaction?
Answers
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1, depending on which rate was used to calculate it.
Determining the rate constantThe rate of the reaction is given by the equation:
Rate = -k[A]
where k is the rate constant and [A] is the concentration of the reactant.
Rate at t=140 s:
Rate = (8.00×10−2 M - 0 M) / (140 s - 0 s)
= 5.71×10−4 M/s
Rate at t=400 s:
Rate = (4.00×10−2 M - 0 M) / (400 s - 0 s)
= 1.00×10−4 M/s
Since this is a zero-order reaction, the rate of the reaction is constant, and we can use either rate to calculate the rate constant:
k = Rate / [A]
Using the rate at t=140 s:
k = 5.71×10−4 M/s / 8.00×10−2 M = 7.14×10−3 s−1
Using the rate at t=400 s:
k = 1.00×10−4 M/s / 4.00×10−2 M
= 2.50×10−3 s−1
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1.
Learn more on zero-order reaction https://brainly.com/question/21663229
#SPJ1
Chemistry Question Help Please!
Use the phase diagram for water to answer the following questions.
35℃ and 85 kPa
-15℃ and 40 kPa
-15℃ and 0.1 kPa
60℃ and 50 kPa
Using the graph above, describe the phase changes that are in equilibrium along each black line. Describe what those equilibria mean.
The boiling point for nitrogen is -195.8℃. Imagine that you had a sealed container of nitrogen gas at room temperature. What are two ways that you could turn the gas into a liquid?

Answers
The two equilibria are the triple point, which is the point when water can exist as ice, liquid, or vapor, and the critical point, which is when water exists as a single phase that has properties of both a liquid and a gas.
What are the triple point and critical point of water?The line connecting the triple point and critical point on the phase diagram of water represents the conditions at which the solid, liquid, and gas phases can coexist in equilibrium.
At the triple point, which occurs at a temperature of 0.01℃ and a pressure of 0.006 atm, the solid, liquid, and gas phases of water exist in equilibrium. This means that at this point, water can exist as ice, liquid, or vapor, depending on the conditions.
At the critical point, which occurs at a temperature of 374℃ and a pressure of 218 atm, the distinction between the liquid and gas phases disappears, and the substance becomes a supercritical fluid. This means that at this point, water exists as a single phase, which exhibits the properties of both a liquid and a gas.
There are two ways to turn nitrogen gas into a liquid:
Cooling: Nitrogen can be cooled down to its boiling point (-195.8°C) using a cryogenic cooler or liquid nitrogen, which causes the gas to condense into a liquid.Increasing pressure: Nitrogen can also be compressed at room temperature to a pressure higher than its vapor pressure, causing it to condense into a liquid.Learn more about the phase diagram of water at: https://brainly.com/question/13518101
#SPJ1
A carbon rod takes up 105 mL of space. With a density of 2.26 g/mL, what
is the mass of the rod? (Do your best to show as much work as possible
like we did on the Pear Decks - if you don't show work or units you will
lose points!)
Your answer
Answers
Hey there!
Mass = ?
volume = 105 mL
Density = 2.26 g/mL
Therefore:
D = m / V
2.26 = m / 105
m = 2.26 * 105
m = 237.3 g
Hope this helps!
When 6 M NaOH is slowly added to a solution containing chromium(III) ion, a precipitate forms. However when an excess of 6 M NaOH is added, the precipitate dissolves, forming a complex ion with a coordination number of four. a. Write the formula of the precipitate.
Answers
Answer:
Cr(OH)3
Explanation:
The addition of 6M sodium hydroxide to a solution that contains chromium(III) ion leads to the net ionic reaction;
3 OH^-(aq) + Cr^3+(aq) -------> Cr(OH)3(s)
Cr(OH)3 is insoluble except an excess of the hydroxide is added then the precipitate dissolves as follows;
Cr^3+(aq) + 4OH^-(aq)------> [Cr(OH)4]^-
Ethylene glycol, C2H6O2, is an odorless, colorless, sweet-tasting liquid used in antifreeze formulations. The antifreeze formulations are 56% ethylene glycol by mass. (Assume the density of the ethylene glycol solution is 1.072 g/mL).How would you prepare 500 mL of 0.350 M solution?
Answers
Step 1 - Finding the concentration in g/L
We know the density of the solution is 1.072 g/ml, which means there are 1.072 g in each ml of solution. This mass corresponds to both water and ethyleneglycol.
Since the percentage in mass of ethyleneglycol is 56%, we can calculate how much ethyleneglycol there is in 1 ml of solution:
\(m_{\text{ethyleneglycol}}=\frac{56}{100}\times1.072=0.600g\)The concentration of ethyleneglycol would be thus 0.6 g/ml.
To convert it to g/L, we just have to multiply it by 1000, because 1L = 1000ml:
\(0.6\times1000=600\text{ g/L}\)The concentration of ethyleneglycol is this solution is thus 600 g/L.
Step 2 - Converting this concentration to mol/L
To convert now to mol/L (M), we just have to divide the concentration by the molar mass of ethyleneglycol (62.07 g/mol):
\(M_{\text{ethyleneglycol}}=\frac{600}{62.07}=9.66\text{ mol/L}\)Note the concentration is a rather high one. We will have to dilute it.
Step 3 - Preparing a 0.350 M solution
We know the final volume of the solution must be 500 ml, and we also know its final concentration (0.350 M). Since we already know its inicial concentration as well, we can use the following formula for dilutions:
\(M_1V_1=M_2V_2\)Plugging the values in the equation:
\(\begin{gathered} 9.66V_1=500\times0.350 \\ \\ V_1=\frac{175}{9.66}=18.11ml \end{gathered}\)Step 4 - Describing how to prepare the solution
As we have calculated in step 3, we would need 18.11 ml of the original solution (9.66 M) for the dilution. We would then add water untill we get the final volume of 500 ml (add 481.89 ml of water)
The resulting solution would be a 500 ml solution of 0.350 M ethyleneglycol.
Answer this please t
Lol

Answers
Answer: trial b
Explanation:
Determine the value of Kc for the following reaction, if the equilibrium concentrations are as follows: [N2]eq = 2.66 M, [H2]eq = 0.64 M, [NH3]eq = 3.34 M.
N2(g) + 3 H2(g) ⇌ 2 NH3(g)
Answers
The value of Kc for the given reaction is 0.0579 (rounded to four decimal places).
The formula for the equilibrium constant, Kc, of a reaction is given by the ratio of the product of the concentrations of the products raised to their respective stoichiometric coefficients to the product of the concentrations of the reactants raised to their respective stoichiometric coefficients.
The stoichiometric coefficients are the coefficients in the balanced chemical equation.
To determine the value of Kc for the reaction given by the following chemical equation:N2(g) + 3 H2(g) ⇌ 2 NH3(g)
we first need to write the expression for Kc.
The expression for Kc is given by the following formula:Kc = [NH3]² / [N2][H2]³.
We are given the equilibrium concentrations as follows:[N2]eq = 2.66 M[H2]eq = 0.64 M[NH3]eq = 3.34 M
We can substitute these values into the expression for Kc and obtain the following:Kc = (3.34)² / (2.66)(0.64)³ = 0.0579 (rounded to four decimal places).
For more such questions on Kc
https://brainly.com/question/15225808
#SPJ8
4) How many grams of carbon dioxide would be needed to produce 3.382 grams of
acetylene (C2H,) using the following reaction?
4 CO2(g) + 2 H20(g) → 2 C2H2(g) +5 02(g)
Answers
Answer:
11.4 g CO₂
Explanation:
Your chemical equation is:
4 CO₂ + 2 H₂O ⇒ 2 C₂H₂ + 5 O₂
You need to produce 3.382 g of acetylene. To find out how many grams of carbon dioxide you need, first convert grams of acetylene to moles using the molar mass. The molar mass of acetylene is 26.04 g/mol.
(3.382 g)/(26.04 g/mol) = 0.130 mol C₂H₂
Now, use the mole ratio between acetylene and carbon dioxide to convert from moles of acetylene to moles of carbon dioxide. You can find the mole ratio by looking at the chemical equation. The mole ratio is (4 mol CO₂)/(2 mol C₂H₂).
(0.130 mol C₂H₂) × (4 mol CO₂)/(2 mol C₂H₂) = 0.260 mol CO₂
Since you now have moles of carbon dioxide, you can convert to grams using the molar mass. The molar mass of carbon dioxide is 44.01 g/mol.
0.260 mol × 44.01 g/mol = 11.4 g CO₂
You will need 11.4 g of CO₂ to produce 3.382 g of C₂H₂.
Identify the species that is oxidized and the species that is reduced in the reaction. Write out half reactions to show how many electrons are gained or lost by each species.
Fe3+(aq) + Al(s) → Al3+(aq) + Fe(s)
Answers
The Fe^3+ is reduced in the reaction while the Al is oxidized in the reaction. The species that loses electrons (is oxidized) is called the reducing agent
What is a redox reaction?A redox reaction (short for reduction-oxidation reaction) is a type of chemical reaction that involves the transfer of electrons between two or more species. In redox reactions, one reactant loses electrons (is oxidized) while another gains electrons (is reduced).
The species that loses electrons (is oxidized) is called the reducing agent, since it causes another species to be reduced. The species that gains electrons (is reduced) is called the oxidizing agent, since it causes another species to be oxidized.
Reduction half equation;
2Fe^3+(aq) + 6e -----> 2Fe(s)
Oxidation half equation;
2Al(s) →2Al3+(aq) + 6e
Learn more about redox reaction:https://brainly.com/question/13293425
#SPJ1
Explain why anhydrous aluminium chloride is fairly soluble in organic solvent while anhydrous magnesium chloride is insoluble
Answers
Answer:
The correct answer is - anhydrous aluminum chloride is covalent whereas anhydrous magnesium chloride is ionic.
Explanation:
Anhydrous aluminum chloride is a covalent compound and we know that covalent compounds have less or no polarity. Organic compounds or solvents are mostly non-polar in nature. And it is a thumb rule that like-dissolves-like.
Thus they dissolve covalent molecules like anhydrous aluminum chloride.
Anhydrous magnesium chloride is an ionic compound that tends to interact with a polar solvent but not in a non-polar solvent such as organic solvents.
Anhydrous aluminum chloride is fairly soluble in the organic solvent while anhydrous magnesium chloride is insoluble because anhydrous aluminum chloride is covalent whereas anhydrous magnesium chloride is ionic.
Why is AlCl3 soluble in organic solvent?AlCl3 quite simply accepts electrons from other atoms, in an try and get a full valence shell of eight electrons. It's why it normally behaves as a Lewis acid. In the response under, the Al atom accepts a lone pair of electrons from a Cl atom.
Why is AlCl3 soluble in water?AlCl3 is hygroscopic and has a great affinity for water. Therefore, aluminum chloride dissolves in water partially.
Learn more about anhydrous aluminum chloride here: https://brainly.com/question/21626996
#SPJ2
What was the purpose of incubating your ubiquity plates upside down?
A.to prevent contaminating
B.to prevent condensation from forming on the agar surface
C. incubating plates upside down is an aseptic technique
D.to prevent organisms from over-growth