what is the wavelength of the photon emitted when the electron in a hydrogen atom makes a transition from the n
Answers
Wavelength of the photon emitted by hydrogen atom when an electron makes a transition from n = 2 to n = 1 state is calculated to be 121.8 nm.
What is wavelength?Wavelength is the distance between identical points in the adjacent cycles of waveform signal propagated in space. In wireless systems, this length is specified in meters , centimeters or millimeters.
Energy of hydrogen atom, En= - 13.6/n
For n= 1
E1= -13.6/1= -13.6 eV
For n= 2
E2= -13.6/2
E2= - 3.4eV
ΔE = E2-E1
= -3.4-(-13.6)
= 10.2 eV
E= hc/λ
As we know, h = 6.626 * 10^-34 J -s, c= 3* 10^8 m/s
10.2 = 6.626 * 10^-34 * 3* 10^8/ λ
λ= 121.8 nm
To know more about wavelength, refer
https://brainly.com/question/10728818
#SPJ4
Note: The question given on the portal is incomplete. Here is the complete question.
Question: The wavelength of the photon emitted by a hydrogen atom when an electron makes a transition from n = 2 to n = 1 state is :
Related Questions
Consider a reaction whose rate constant is 3. 4 m-1s-1 at 600k and 31. 0 m-1s-1 at 750k. Find the activation energy (in kj/mol) of the reaction. Express your answer to 2 decimal places
Answers
The activation energy of the reaction is approximately 71.46 kJ/mol, rounded to 2 decimal places.
To find the activation energy (Ea) of the reaction, we can use the Arrhenius equation, which relates the rate constant (k) to the temperature (T) and activation energy. The Arrhenius equation is given by:
k = A * e^(-Ea/RT)
Where:
k is the rate constant
A is the pre-exponential factor (frequency factor)
Ea is the activation energy
R is the gas constant (8.314 J/(mol·K))
T is the temperature in Kelvin
We have two sets of data:
At 600 K, k1 = 3.4 m^(-1)s^(-1)
At 750 K, k2 = 31.0 m^(-1)s^(-1)
Taking the natural logarithm (ln) of both sides of the Arrhenius equation, we can rearrange the equation to solve for the activation energy:
ln(k) = ln(A) - (Ea/RT)
We can create two equations using the given data:
ln(k1) = ln(A) - (Ea/(R * 600))
ln(k2) = ln(A) - (Ea/(R * 750))
Subtracting the second equation from the first eliminates the ln(A) term:
ln(k1) - ln(k2) = (Ea/R) * ((1/600) - (1/750))
Simplifying further:
ln(k1/k2) = (Ea/R) * ((750 - 600) / (600 * 750))
Now we can solve for Ea:
Ea = (R * (ln(k1/k2))) / ((750 - 600) / (600 * 750))
Using the given values and the appropriate units:
Ea = (8.314 J/(mol·K) * ln(3.4/31.0)) / ((750 - 600) / (600 * 750))
Converting the units from J to kJ:
Ea = (8.314 × 10^(-3) kJ/(mol·K) * ln(3.4/31.0)) / ((750 - 600) / (600 * 750))
Ea ≈ 71.46 kJ/mol
Therefore, the activation energy of the reaction is approximately 71.46 kJ/mol, rounded to 2 decimal places.
learn more about activation energy here
https://brainly.com/question/28384644
#SPJ11
draw the full mechanism (arrow-pushing) for the acid-base reaction between triethanolamine and stearic acid.
Answers
The acid-base reaction between triethanolamine and stearic acid involves the deprotonation of stearic acid by triethanolamine.
The amine group in triethanolamine acts as a base and abstracts a proton from the carboxylic acid group in stearic acid. This forms a carboxylate ion and a protonated triethanolamine molecule. Triethanolamine (TEA) is a tertiary amine with three hydroxyl groups. Stearic acid is a long-chain carboxylic acid. In the reaction, one of the hydroxyl groups in TEA acts as a base and deprotonates the carboxylic acid group in stearic acid. The lone pair of electrons on the nitrogen atom in TEA attacks the proton of the carboxylic acid group, breaking the O-H bond and forming a new C-N bond. This results in the formation of a carboxylate ion, where the oxygen of the carboxylic acid group gains a negative charge, and a protonated triethanolamine molecule, where the nitrogen gains a positive charge. The reaction can be represented using arrow-pushing notation to show the movement of electrons throughout the process.
Learn more about triethanolamine here:
https://brainly.com/question/3189819
#SPJ11
What volume is occupied by 18.4 g oxygen at 28.0°C and a pressure of 0.998 torr? (round to sig figs)
Answers
Answer:
11.0 dm³
Explanation:
From the question,
Applying
PV= nRT............... Equation 1
Where P = pressure of oxygen gas, V = volume of oxygen gas, n = number of moles of oxygen, R = molar constant, T = Temperature.
make V the subeject of the equation
V = nRT/P............. Equation 2
But,
Number of mole (n) = Mass of oxygen(m)/Molar mass of oxygen(m')
n = m/m'....................... Equation 3
Substitute equation 3 into equation 2
V = mRT/Pm'............. Equation 4
Given: T = 28°C = (28+273) = 301 K, P = 0.998 torr = (0.998×0.00131579) = 1.3132 atm, m = 18.4 g
Constant: R = 0.082 atm.dm³/K.mol, m' = 32 g/mol.
Substitute these values into equation 4
V = (301×18.4×0.082)/(32×1.3132)
V = 454.1488/42.0224
V = 10.81 dm³
V = 11.0 dm³
Which species will have the strongest mass shift on a magnetic susceptibility balance?.
Answers
O2 is the correct answer.
Explanation:
On a magnetic susceptibility balance, the O2 species will have the strongest mass shift since stronger paramagnetic species will have a larger mass shift.
The oxygen atoms in the O2 species are paramagnetic because unpaired electrons rotate in the same direction, increasing the magnetic field force. As a result, the oxygen atoms with two unpaired electrons will exhibit the largest mass shift on a magnetic susceptibility balance.
The magnitude of the mass shift is -O2, which increases with species paramagneticity. The mass shift increases with species paramagneticity. Therefore, on a magnetic susceptibility balance, oxygen will have the highest mass shift since it has two unpaired electrons in the molecular orbital diagram.
To know more about magnetic susceptibility balance visit:-
https://brainly.com/question/2285863
#SPJ4
A chemical reaction that has the general formula of AB + C → CB + A is best classified as a
synthesis
polymerization
decomposition
oxidation
replacement
reaction.
Answers
Replacement reaction.
Further explanationGiven
A chemical reaction
AB + C → CB + A
Required
reaction type
Solution
There are several types of reactions that can occur
synthesis: 2 elements combine to form a single product
polymerization: monomers combine to produce a polymer
decomposition: One compound breaks down into 2 components
oxidation : a change in oxidation number
replacement can be divided into 2
single replacement if one element replaces the other elements of a compound
double replacement if there is an ion exchange between two ion compounds in the reactant to form two new ion compounds
AB + C → CB + A
If we look at this reaction, element C replaces element A to form a new compound CB. So it is single replacement. But this reaction can also be said to be an oxidation-reduction reaction because there is a change in oxidation number
element A undergoes a reduction reaction
element C undergoes an oxidation reaction
So types of redox reactions can be combination, decomposition, displacement, combustion, and disproportion.
Answer:
oxidation
Explanation:
i passed and got 100%
2. An atom of Be has four protons, five neutrons and four electrons. What 10 poin
is the mas of Be? *
O a.4
b. 5
c. 1
09
Answers
Answer:
Five ( 5 ) is the correct answer
What volume of 3.00M NaCl is required for a
reaction that requires 146.3g of NaCl?
Answers
Answer: 0.834 L
Explanation:
Volume is the scalar quantity that is the space required by the solution or the substance. The volume required for the 146.3g of 3.00M NaCl is 0.834 L.
What is the relationship between volume and molarity?Molarity is the concentration of the solution and is given as the ratio of the moles and the volume of the solution. The volume of the solution or the solute is inversely proportional to the molarity of the substance.
Molarity is given as,
\(\rm Molarity (M) =\rm \dfrac{ Moles(n) }{\text{Volume in L}}\)
Here,
Molarity of sodium chloride = 3.00M
Mass of sodium chloride = 146.3g
Molar mass of sodium chloride = 58.44 g/mol
Substituting values in the equation:
\(\begin{aligned} \rm Molarity &= \rm \dfrac{mass}{Molar \;mass \times Volume}\\\\\rm V &= \dfrac{146.3}{ 58.44 \times 3.00}\\\\&= 0.8344 \;\rm L\end{aligned}\)
Therefore, the volume of the sodium chloride required is 0.834 L
Learn more about molarity and volume here:
https://brainly.com/question/12127540
If two wave crests overlap each other and one has an amplitude of 5 and the other has an amplitude of 3, what is the interference that is created?
Answers
Answer:
The interference is 8
Explanation:
which of the following describes the reaction of molecules as snow melts
a) The ice absorbs heat energy and the molecules move further away
b) The ice releases heat energy and the molecules move further away
c) The ice absorbs heat energy and the molecules move closer together
d) The ice releases heat energy and the molecules move closer together
Answers
During the melting of ice, the ice absorbs heat energy and the molecules move further away; option A.
What is melting?Melting refers to the process by which a solid substance changes to liquid when heat is added to it.
The melting of pure substances occur at a definite temperature called the melting point of that substance.
The molecules of a substance move further apart when they melt as the attractive forces between them are weakened.
The melting of ice is an example of the process of melting.
During the melting of ice, the ice absorbs heat energy and the molecules move further away.
In conclusion, melting of solids occur when heat is added to the solid.
Learn more about melting at: https://brainly.com/question/40140
#SPJ1
Label the different parts of the atom.

Answers
Answer:(a) electrons
(b) nucleus
(c) protons
(d) neutrons
(e) mass number
Explanation:
Explain why it’s important in determining the geometry of a molecule to know about both bonds AND lone pairs on the central atom?
Answers
Answer:
Using the VSEPR theory, the electron bond pairs and lone pairs on the center atom will help us predict the shape of a molecule. The shape of a molecule is determined by the location of the nuclei and its electrons. The electrons and the nuclei settle into positions that minimize repulsion and maximize attraction.
Explanation:
this from the internet
sorry if im wrong
The lone pairs and bond pairs in a molecule are used to determine the location of electron pair geometry around the molecule.
What is electron pair geometry?The geometry of a molecule refers to the shape of the molecule. The geometry of a molecule is determined by the valence shell electron pair repulsion theory.
Now, we know that both lone pairs and bond pairs in the molecule are used to determine the electron domain geometry around the central atom as they take the most appropriate orientations in space to minimize electron pair repulsions.
Learn kore about electron pair geometry: https://brainly.com/question/365923
In the Millikan oil droplet experiment, the oil is sprayed from an atomizer into a chamber. The droplets are allowed to pass through the hole into the chamber so that their fall can be observed. The top and bottom of the chamber consist of electrically charged plates. The upper plate is positively charged, and the lower plate is negatively charged. X rays are introduced into the chamber so that when they strike the oil droplets, the droplets will acquire one or more negative charges. The electric field (voltage) is applied to the metal plates.
Watch the animation and identify the effects of an electric field on the motion of a negatively charged oil droplet. Consider the gravitational force as Fg and the electric force as Fe. All the other forces acting on the oil droplet can be ignored as their effect on the motion of the oil droplet is negligible.
A/ In the absence of an electric field, the oil droplet falls freely due to the gravitational force.
B/ If Fe is increased until it is equal to Fg, the negatively charged oil droplet will remain stationary.
C/ If Fe is greater than Fg, the negatively charged oil droplet will move freely toward the negatively charged plate.
D/ In the presence of an electric field, the negatively charged oil droplet moves freely toward the negatively charged plate.
** I chose B, but that was the wrong answer
Answers
C/ If Fe is greater than Fg, the negatively charged oil droplet will move freely toward the negatively charged plate.
In the Millikan oil droplet experiment, the negatively charged oil droplets are subjected to an electric field created by the charged plates. The electric force (Fe) acts on the oil droplet in a direction opposite to the gravitational force (Fg). When Fe is greater than Fg, the electric force overcomes the gravitational force, causing the negatively charged oil droplet to experience an upward force. As a result, the oil droplet moves freely upward toward the negatively charged plate.
Option B is incorrect because if Fe is equal to Fg, the forces balance each other, resulting in a stationary droplet. However, the question states that Fe is increased until it is greater than Fg, implying that the droplet is no longer stationary but moves in response to the electric force.
Therefore, option C is the correct answer, as it describes the effect of an electric field on the motion of a negatively charged oil droplet in the Millikan oil droplet experiment.
To learn more about Millikan oil droplet experiment, here
https://brainly.com/question/32330429
#SPJ4
by titration, it is found that 12.5 ml of 0.169 m naoh(aq) is needed to neutralize 25.0 ml of hcl(aq). calculate the concentration of the hcl solution.
Answers
The concentration of the HCl solution is calculated to be 0.0845 M using the formula M1V1 = M2V2.
The concentration of the HCl solution can be calculated using the formula M1V1 = M2V2, where M1 is the concentration of the NaOH solution, V1 is the volume of the NaOH solution, M2 is the concentration of the HCl solution, and V2 is the volume of the HCl solution.
Given:
M1 = 0.169 M
V1 = 12.5 mL
V2 = 25.0 mL
We need to find M2.
Rearranging the formula to solve for M2 gives us:
M2 = (M1V1)/V2
Substituting the given values into the formula gives us:
M2 = (0.169 M)(12.5 mL)/(25.0 mL)
M2 = 0.0845 M
Therefore, the concentration of the HCl solution is 0.0845 M.
To learn more about titration, refer:
https://brainly.com/question/12483812#
#SPJ11
Convert the following 3grams = milligrams
Answers
Answer:
3000 milligrams
Explanation:
to get milligrams you add 3 zero at the end
select all of the following types of alkyl halides that are capable of forming a carbocation.
Answers
The types of alkyl halides that are capable of forming a carbocation include:
1. Primary alkyl halides (1°): These have the halogen atom bonded to a carbon that is bonded to only one other carbon atom.
2. Secondary alkyl halides (2°): These have the halogen atom bonded to a carbon that is bonded to two other carbon atoms.
3. Tertiary alkyl halides (3°): These have the halogen atom bonded to a carbon that is bonded to three other carbon atoms.
Tertiary alkyl halides are the most capable of forming carbocations due to their greater stability, followed by secondary and primary alkyl halides.
Learn more about carbocation:
https://brainly.com/question/11486868
#SPJ11
What is the name of this ?

Answers
3-ethyl-4,5-dimethyl-octane
A bicycle weighs 780. pounds. If the pressure exerted by its
tires on the ground is 30.5 pounds per square centimeter, what
is the area of one of the two tires that's in contact with the
road? (This will be half as much as the pressure exerted by
both tires.)
Answers
Answer:it’s 12.8cm^2 and I saw that last anwser and he’s not wrong but yeah I had trouble figuring it out but it’s just force divided by area for both tires multiply that by 2
Explanation:
A bicycle weighs 780. pounds. If the pressure exerted by its tires on the ground is 30.5 pounds per square centimeter, the area of one of the two tires that's in contact with the road is 12.8cm².it’s just force divided by area for both tires multiply that by 2.
What is pressure ?Pressure is defined as the force applied perpendicular to an object's surface per unit area over which that force is distributed. Gauge pressure is the pressure in relation to the surrounding atmosphere. Pressure is expressed using a variety of units.
Pressure is defined as force per unit area of surface; the SI unit of pressure is the pascal (Pa), which is defined as one newton per square metre (N/m2). An object's pressure is proportional to the force it exerts and inversely proportional to the area over which the force is exerted.
The physical force exerted on an object is defined as pressure. The force applied per unit area is perpendicular to the surface of the objects. F/A is the basic pressure formula (Force per unit area).
Thus, the area of one of the two tires that's in contact with the road is 12.8cm².
To learn more about the pressure, follow the link;
https://brainly.com/question/29341536
#SPJ2
What is the empirical formula of compund that contains 4. 03g of hydrogen per mole, 64. 14 g of sulfur per mole and 128 g of oxyen per mole
Answers
The empirical formula of compound that contains 4. 03g of hydrogen per mole, 64. 14 g of sulfur per mole and 128 g of oxygen per mole is H₂SO₂.
given that :
mass of hydrogen = 4.03 g
mass of sulfur = 64.14 g
mass of oxygen = 128 g
moles of hydrogen = mass / molar mass
= 4.03 / 1
= 4mol
moles of sulfur = 64.14 / 32
= 2 mol
moles of oxygen = 128 / 32
= 4 mol
dividing by the smallest one , we get
mole of H = 2
mole of S = 1
mole of O = 2
The empirical formula = H₂SO₂
Thus, the empirical formula of compound that contains 4. 03g of hydrogen per mole, 64. 14 g of sulfur per mole and 128 g of oxygen per mole is H₂SO₂.
To learn more about empirical formula here
https://brainly.com/question/25780603
#SPJ4
Balancing chemical equation:
_Na+_MgF2—>_NaF+_Mg
Na= ? Na= ?
Mg= ? Mg= ?
F= ? F= ?
Answers
2Na + MgF2 → 2NaF + Mg
What type of channel is affected by tetrodotoxin (TTX)?
TTX blocks the Na+ channel by binding tightly to a specific site on the outside of the channel.
Answers
Tetrodotoxin (TTX) specifically affects voltage-gated sodium channels.
These channels are responsible for the generation and propagation of action potentials in excitable cells, including neurons and muscle cells. TTX binds tightly to a specific site on the outside of the sodium channel, blocking the movement of sodium ions through the channel pore.
By blocking sodium channels, TTX prevents the influx of sodium ions into cells during depolarization, effectively inhibiting the generation and propagation of action potentials. This leads to the disruption of normal electrical signaling in excitable tissues, resulting in various physiological effects depending on the affected tissues.
Due to its potent inhibitory effects on sodium channels, TTX is known for its use as a toxin, primarily found in pufferfish and certain other marine organisms. Ingesting TTX-contaminated seafood can lead to severe poisoning, characterized by paralysis, respiratory failure, and potentially fatal consequences.
Research on TTX and its interactions with sodium channels has also provided valuable insights into the function and structure of these channels, contributing to our understanding of electrical signaling in cells and the development of drugs targeting sodium channels for therapeutic purposes.
Learn more about Tetrodotoxin from the link given below.
https://brainly.com/question/2927710
#SPJ4
How will the following stresses affect this equilibrium system (iron thiocyanate system): Fe+3 {pale yellow} + SCN- FeSCN+2 {red} + heatA) Add KSCN (a source of adding SCN- ions in solution)?

Answers
ANSWER
The addition of KSCN to the system will shift the arrow to the product sides
EXPLANATION
Firstly, we need to define the term chemical equilibrium.
Chemical equilibrium can be defined as the state in which the reactants and the products are present in concentration and do not have a tendency to change with time.
To determine the shift of the chemical equilibrium in the reaction provided, state the le Chatelier's principle
Le Chatelier's principle states that when external constraints such as temperature, pressure, concentration, etc system in chemical equilibrium, the equilibrium arrow shifts so as to annul the effect of the external constraints.
From the reaction provided
\(\text{ Fe}^{+3}\text{ + SCN}^-\text{ }\rightleftarrows\text{ FeSCN}^{+2}\text{ + heat}\)The above equation shows that the reaction is an exothermic reaction because heat is liberated to the surroundings.
When one of the reactants is supplied to the system constantly, more of its product is formed. Hence, the equilibrium arrow shifts to the right-hand side of the reaction.
Hence, the addition of KSCN to the system will shifts the arrow to the product sides
g a 0.1599 gram sample containing an unknown amount of chloride is titrated with a 0.7890 m silver nitrate solution. 30.83 ml of the silver nitrate solution was required to reach the end point of the titration. what is the mass percent of chloride in the original sample?
Answers
To determine the mass percent of chloride in the original sample, we need to first calculate the amount of chloride present in the sample. We can do this by using the balanced equation for the reaction between chloride and silver nitrate:
AgNO3 + Cl- → AgCl + NO3-
First, we need to calculate the number of moles of silver nitrate used in the titration:
moles of AgNO3 = volume (L) x molarity (M) = 0.03083 L x 0.7890 M = 0.02439 moles
Next, we can use the balanced equation to find the number of moles of chloride:
moles of Cl- = moles of AgNO3 = 0.02439 moles
Finally, we can use the number of moles of chloride to calculate the mass of chloride in the sample:
mass of Cl- = moles of Cl- x molar mass of Cl- = 0.02439 moles x 35.5 g/mol = 0.868 g
To find the mass percent of chloride in the original sample, we divide the mass of chloride by the total mass of the sample and multiply by 100%.
mass percent of Cl- = (mass of Cl- / total mass of sample) x 100%
Since we don't know the total mass of the sample, we can use the mass of chloride we just calculated and the formula above to find the mass percent of chloride in the original sample.
mass percent of Cl- = (0.868 g / 0.1599 g) x 100% = 54.3%
So, the mass percent of chloride in the original sample is 54.3%.
To learn more about mass percent:
https://brainly.com/question/26150306
#SPJ4
In California, the Pacific plate slides past the North American plate. If the Pacific plate is moving at a speed of 5 centimeters per year, how long will it take for the plate to travel 100 meters?
Answers
Answer:
500
Explanation:
sddds2
convert 0.650 atm of pressure to its equivalent in millimeters of mercury. express the pressure numerically in millimeters of mercury.
Answers
The pressure of 0.650 atm numerically in milimeters of mercury is 494 mmHg. 1 atm is equivalent to 760 mmHg.
What are equivalent standard pressures?There are several pressure units. They are atm, mmHg, torr, Pa, and psi. The equivalent standard pressures are as follows:
1 atm = 760 mmHg = 760 torr = 101,325 Pa = 14.7 psi
Atm (atmosphere) is defined as the atmospheric pressure at sea level.mmHg (milimeters of mercury) is defined as the amount of pressure exerted by a 1 mm high column of mercury.torr is the unit named after Evangelista Torricelli, the inventor of mercury barometer. It's equivalent to mmHg.Pa (Pascal) is the standard unit of pressure.psi (pounds per square inch) is defined as the pound-force applied to an area of 1 square inch.What is 0.650 atm of pressure if it is converted to milimeters of mercury?
1 atm = 760 mmHg
0.650 atm = 0.650 × 760 mmHg
0.650 atm = 494 mmHg
Hence, the pressure numerically in milimeters of mercury is 494 mmHg.
Learn more about pressure unit here:
brainly.com/question/17327541
#SPJ4
An atom that has one or two valence electrons is most likely to be - Nonreactive only
A) with nonmetals
B) Reactive with metals
C) Highly reactive
D) nonreactive
Answers
Answer:
C) Highly reactive
Explanation:
An atom with one or two valence electrons more than a closed shell is highly reactive, because the extra valence electrons are easily removed to form a positive ion
According to the electronic configuration, an atom that has one or two valence electrons is most likely to be highly reactive as it can easily loose 1 or 2 electrons.
What is electronic configuration?Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/29757010
#SPJ6
how many helium atoms have the same mass as 1 sulfur
Answers
3. What makes water a polar covalent compound?
Answers
Answer:
Water is a polar covalent compound since it has Oxygen and Hydrogen which are 2 non-metals and the atoms have an unequal distribution of charge. Oxygen has δ- since it is more electronegative and Hydrogen has δ+. The shape of the water molecule is not symmetrical and dipoles are cancelled out making it a polar covalent compound.
Answer:
it is colourless
Explanation:
its tastleś
The pH of solution A is 7 and the pH of solution B is 5. Solution A has ____ times ______ hydrogen ion concentration compared to solution B.
Answers
Answer: 100, less
Explanation:
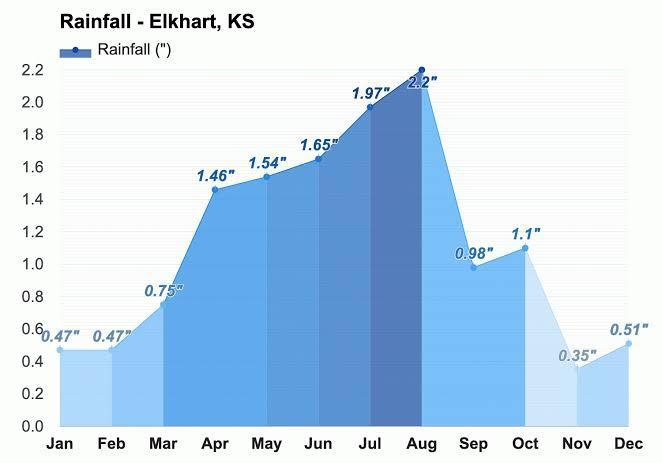
create a bar graph that shows the total yearly precipitation for elkhart kansas
Answers
The total yearly precipitation for elkhart kansas is attached in the form of graph below.
What is precipitation?Precipitation is defined as any liquid or frozen water which forms in the atmosphere and then gets received on Earth.It is one of the most important steps of the water cycle.
Precipitation takes place in form of clouds when water vapor gets accumulated in clouds and they get bigger and heavy, when the clouds become heavy enough they fall to the land in the form of rain.f a cloud is present at higher altitudes , the water present in the clouds freezes and fall to the ground in form of snow,hail.
Learn more about precipitation ,here:
https://brainly.com/question/18109776
#SPJ1
The sentence below from the section "1 Million Species Threatened" helps prove the claim that humans need nature.
"Nature is essential for human existence and good quality of life," the report said.
Which selection from the section provides further support for the claim?
3 points
A big report released on May 6 warned that nature is in trouble and estimated that 1 million species are threatened with extinction if nothing is done.
Food, energy, medicine, water, protection from storms and floods and slowing climate change are some of the 18 ways nature helps keep people alive, the report said.
He pointed to how difficult it has been for China to come back from decades of forest loss. The country has replanted trees in recent years.
The Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services report points to more than 2,500 wars and other conflicts over fossil fuels, water, food and land.
Answers
The selection from the section that provides further support for the claim is that B. Food, energy, medicine, water, protection from storms and floods and slowing climate change are some of the 18 ways nature helps keep people alive, the report said.
What is the selection that supports the claim?Selection 2, "Food, energy, medicine, water, protection from storms and floods and slowing climate change are some of the 18 ways nature helps keep people alive, the report said." provides further support for the claim that humans need nature.
It lists several specific ways in which nature is essential for human existence and good quality of life, and it is align with the statement "Nature is essential for human existence and good quality of life," the report said. Additionally, it provides an estimation of 18 ways which nature helps human to survive, adding more weight on the statement.
Learn more about claim on:
https://brainly.com/question/2748145
#SPJ1