What mass of carbon dioxide is formed in the combustion of 78. 7 g of acetone?.
Answers
In order to calculate the mass of carbon dioxide formed during the combustion of acetone, we need to balance the chemical equation first. Combustion of acetone takes place in the presence of oxygen gas to produce carbon dioxide and water.
The balanced chemical equation is given as:2C3H6O + 9O2 → 6CO2 + 6H2OFrom the equation, we can see that for every 2 moles of acetone burned, 6 moles of carbon dioxide are produced. Now, we need to calculate the number of moles of acetone present in 78.7 g.
To do this, we divide the mass of acetone by its molar mass: Molar mass of acetone (C3H6O) = 3 x 12.01 + 6 x 1.01 + 16.00 = 58.08 g/mol Number of moles of acetone = mass / molar mass = 78.7 / 58.08 = 1.354 moles Now that we know the number of moles of acetone, we can calculate the moles of carbon dioxide formed.
To know more about carbon visit:
https://brainly.com/question/13046593
#SPJ11
Related Questions
describe a procedure to remove the water from the mixture that passes through the filter and collects in the beaker.
Answers
Answer:
The procedure is Filtration
Explanation:
Filtration is a process that is use to separate insoluble solid from liquid for example Salt and water.
This process uses filter paper which is placed inside a funnel and then placed inside a beaker.
The mixture of sand and water is poured into the the filter paper and water then drains out down to the funnel and the beaker.
The solid part remain at the filter paper and it is called the residue and the water is the filtrate.
What would the final product beaker look like for when nitric acid reacts with strontium hydroxide?
Answers
The final product beaker for the reaction between nitric acid and strontium hydroxide would contain strontium nitrate and water. [Precipitation] of strontium nitrate would occur, while water would remain in the solution. The beaker may also contain some excess nitric acid if it was not completely neutralized by strontium hydroxide.
When nitric acid (HNO3) reacts with strontium hydroxide (Sr(OH)2), a neutralization reaction occurs. The balanced chemical equation for this reaction is:
2HNO3 + Sr(OH)2 -> Sr(NO3)2 + 2H2O
In this reaction, the hydrogen ions (H+) from nitric acid react with the hydroxide ions (OH-) from strontium hydroxide to form water (H2O). The remaining components, strontium ions (Sr2+) and nitrate ions (NO3-), combine to form strontium nitrate (Sr(NO3)2), which is a soluble salt.
In the final product beaker, the strontium nitrate would be in the form of dissolved ions since it is soluble in water. Water would be present in the solution as the solvent. If there was any excess nitric acid that was not completely neutralized, it would also be present in the beaker.
Learn more about strontium nitrate from the given link: https://brainly.com/question/22881954
#SPJ11
The rock shown below is in California's Death Valley
National Park.
Whích phrase most likely describes the environment in which this rock
formed?
A. Low mineral content
B. Low temperature
C. High humidity
D. High pressure
Answers
D. High pressure most likely describes the environment in which this rock formed.
The rock shown in the photograph is a type of sedimentary rock called "conglomerate." Conglomerate rocks are formed by the deposition and cementation of large, rounded fragments of pre-existing rocks. The rounded nature of the rock fragments suggests that they have undergone transport and erosion before being deposited and lithified into conglomerate.
The most likely environment for the formation of conglomerate rocks is a high-energy setting, such as river channels, where the rock fragments are subjected to high pressures during transport. The high pressure is a result of the weight and force of the moving water, which helps to compact and cement the rock fragments together.
The other options, such as low mineral content, low temperature, and high humidity, are not necessarily indicative of the environment in which conglomerate rocks are formed. Conglomerate formation is primarily associated with the physical processes of transportation and deposition, rather than the chemical composition, temperature, or humidity of the environment.
To learn more about conglomerate, here
https://brainly.com/question/7945792
#SPJ4

PLEASE HELP IM FAILING AND TODAY IS THE LAST DAY!! PICTURE PROVIDED!!
In your graph, which variable went on the x-axis? Which variable went on the y-axis? Explain why for both.

Answers
Answer:
Since the trials depend on the number of shakes, trials is the DEPENDENT VARIABLE and shakes is the INDEPENDENT VARIABLE as something depends on it.
Independent is on the X axis
Dependent is on the Y axis
An electrochemical cell used for the "Quant" purpose (that is, to find unknown concentration of the analyte) is based on: A. a battery B. an electrolytic cell C. neither A nor B D. either A or B E. can not be decided
Answers
The answer to your question is D, either A or B. An electrochemical cell can be used for quantitative analysis, also known as "quant" analysis, to determine the concentration of an unknown analyte.
Both batteries and electrolytic cells can be used for this purpose, depending on the specific setup of the electrochemical cell. Therefore, the answer is that it could be either A or B.
An electrochemical cell used for the "Quant" purpose (that is, to find unknown concentration of the analyte) is based on: C. neither A nor B. It is actually based on a galvanic cell or a potentiometric cell, which measure the potential difference between two half-cells in order to determine the concentration of the analyte.
To know mor about electrolytic cell visit:
https://brainly.com/question/4030224
#SPJ11
Craik and Lockhart (1972) proposed the idea that deeper levels of ____ results in stronger retention of memories
A. Rehearsal
B. Multitasking
C. Memory
D. Processing
Answers
Answer: D. Processing
Explanation: 100% on topic test also its in my e notes.

Craik and Lockhart has proposed theory of memory processing. Thus, option D is correct.
Craik and Lockhart proposed the theory for the processing of the level of memory. The memory in humans can be sensory memory, working memory, and long-term memory.
The long term memories have been stored in the deeper cells and the increased or strong stimulation of the processing results in the stronger retention of the memories. Thus, option D is correct.
For more information about the memory processing, refer to the link:
https://brainly.com/question/6249980
can any one solve this quiz plz

Answers
Answer:
1. It is stoichiometric.
2. O2 is the limiting reactant.
3. 9.0 g of C2H6 remain unreacted.
4. 17.6 g of CO2.
5. 85.2%.
Explanation:
Hello there!
In this case, for the given chemical reaction:
\(2C_2H_6+7O_2\rightarrow 4CO_2+6H_2O\)
We can see that:
1. It is stoichiometric and is balanced because the reactants yields the products according to the law of conservation of mass.
2. In this part, it is possible to calculate the moles of ethane by using its molar mass:
\(n_{C_2H_6}=15g*\frac{1molC_2H_6}{30.08g} =0.50molC_2H_6\)
And the moles of oxygen by knowing that one mole is contained in 22.4 L at STP:
\(n_{O_2}=\frac{1mol}{22.4L} *15.68L=0.7molO_2\)
Thus, by calculating the moles of carbon dioxide product by each reactant, we can identify the limiting one:
\(n_{CO_2}^{by\ C_2H_6}=0.50molC_2H_6*\frac{4molCO_2}{2molC_2H_6} =1.0molCO_2\\\\n_{CO_2}^{by\ O_2}=0.70molO_2*\frac{4molCO_2}{7molO_2} =0.4molCO_2\\\)
Thus, since oxygen yields the fewest moles of CO2 product, we infer it is the limiting reactant.
3. In this part, we calculate the mass of C2H6 that actually react first:
\(m_{C_2H_6}^{reacted}=0.4molCO_2*\frac{2molC_2H_6}{4molCO_2}*\frac{30.08gC_2H_6}{1molC_2H_6} =6.0gC_2H_6\)
Thus, the leftover of ethane (C2H6) as the excess reactant is:
\(m_{C_2H_6 }^{leftover}=15g-6.0g=9.0g6.0C_2H_6\)
4. Since 0.4 moles of carbon dioxide were produced, we use its molar mass to calculate the mass as its theoretical yield:
\(m_{O_2}^{theoretical}=0.4molCO_2*\frac{44gCO_2}{1molCO_2}=17.6gCO_2\)
5. Finally, the percent yield is gotten by dividing the actual yield by the theoretical one:
\(Y=\frac{15g}{17.6}*100\%\\\\Y=85.2\%\)
Best regards!
3.Zinc has an atomic number of 30 and a mass number of 65, how many neutrons does it have?
30
65
35
95
Answers
Answer:
35
Explanation:
Given parameters:
Atomic number of zinc = 30
Mass number of zinc = 65
Unknown
Number of neutrons = ?
Solution:
The mass number of an atom is the sum of its protons and neutrons;
Mass number = Protons + neutrons
Atomic number is the number of protons in an atom;
Atomic number = Protons
So;
Mass number = Atomic number + Neutrons
Neutrons = Mass number - Atomic number
Neutrons = 65 - 30 = 35
The number of neutrons in the atom is 35
7. What happens to the electrons in the metal electrode atoms when high voltage is applied to the CRT
electrodes?
Answers
Answer:
Electrons accelerated to high velocities travel in straight lines through an empty cathode ray tube and strike the glass wall of the tube, causing excited atoms to fluoresce or glow.
Explanation:
solid aluminum al and oxygen o2 gas react to form solid aluminum oxide al2o3. suppose you have 2.0 mol of al and 3.0 mol of o2 in a reactor. what would be the limiting reactant? enter its chemical formula below.
Answers
The limiting reactant for solid aluminum al and oxygen o2 gas react to form solid aluminum oxide al2o3. suppose you have 2.0 mol of al and 3.0 mol of o2 in a reaction is Al.
For given trouble chemical response will be,4Al+3O2 ⇒2Al2O3By the way of means of above stability response it's miles that four mole Al will react with three mole O2 for whole response. i.e. Al:O2= four: three so four mole Al at = three mole O2 therefore, 1 mole Al will eat= three/four mole of O21 mole Al will eat = 0.75 mole of O2 right here O2 is given 2 moles therefore Al will eat first via way of means of reacting with 0.75 mole of O2. So Al is restricting or limiting reagent for the solid aluminum al and oxygen o2 gas react to form solid aluminum oxide al2o3.
Read more about the solvent:
https://brainly.com/question/25326161
#SPJ4
What is the difference between an independent variable and a dependent variable in an experiment?
Answer in a complete sentence or use the sentence frame below
The difference between an independent variable and a dependent variable is an independent variable is ___________ and a dependent variable is ______________.
Answers
The difference between an independent variable and a dependent variable is an independent variable is the variable that is manipulated by the experimenter, and a dependent variable is the variable that is measured to see if it is affected by the independent variable.
In statistics, an independent variable is a variable that is manipulated or controlled by the researcher to observe its effect on a dependent variable.
Learn more about independent variable at:
https://brainly.com/question/29430246
#SPJ1
The independent variable in an experiment is the factor that researchers deliberately change to test its effects, while the dependent variable is the factor that they measure to see if it changes in response to the manipulation of the independent variable.
Explanation:The difference between an independent variable and a dependent variable is that an independent variable is a factor in an experiment that the researcher manipulatively changes to see if it has any effect, while a dependent variable is the factor the researcher measures to see if it changes as a result of the manipulation of the independent variable.
For example, if you were running an experiment to see if different amounts of sunlight affected the rate at which a plant grows, the independent variable would be the amount of sunlight the plant receives (because you, the researcher, are changing it), and the dependent variable would be the growth rate of the plant (because you are measuring this to see if it changes in response to the changing amount of sunlight).
Learn more about Independent and Dependent Variables here:https://brainly.com/question/38097299
#SPJ12
Find the concentration of a solution that is prepared by mixing 15 g of sugar
and 125 ml of water.
Answers
Answer: Use the formula x = (c ÷ V) × 100 to convert the concentration (c) and volume (V) of the final solution to a percentage. In the example, c = 60 ml and V = 350 ml.
Explanation: Divide the mass of the solute by the total volume of the solution. Write out the equation C = m/V, where m is the mass of the solute and V is the total volume of the solution. Plug in the values you found for the mass and volume, and divide them to find the concentration of your solution.
A student has measured the volume of a sample of hydrogen gas and has found that
she has a total of 15.84 moles of the gas. What is the mass of this sample of
hydrogen gas? Please round your answer to two digits after the decimal point, and
remember to include correct, complete units (including substance formula).
Answers
The correct answer is 2.03×1/10² mol
500 cm³ = 0.5 L,760 mm of Hg=1 atm.
Now apply the ideal gas law, n= PV/RT
n= 1×0.5/ 0.0821×300
⟹n=2.03×10 ^−2 mol
What is the ideal gas law?
It is also called the general gas equation, is the equation of state for a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's Law, Charles' Law, Avogadro's Law, and Gay-Lussac's Law.To know more about ideal gas law, click the link given below:
https://brainly.com/question/28257995
#SPJ1
a total of 1.4 moles of sodium nitrate is dissolved in enough water to make 2.0 liters of an aqueous solution. the gram-formula mass of sodim notrate is 85 grams per mole. determine the molarity of the solution
Answers
Answer:
1.4 moles/ 2.0 L= 0.7 M
Explanation: Molarity= moles of solute/ Liters of solution
therefore just plug the numbers in and you'll find the molarity to equal. 0.7
Which statement is best supported by the information in the chart?
Atom X will donate electrons to Atom Y.
Atom X will accept electrons from Atom Y.
Atom Y will accept electrons from Atom Z.
Atom Z will donate electrons to Atom X.
Answers
Answer:
B
Explanation:
Because , it is the answer .
Based on the information provided in the chart, the statement that is best supported is Atom X will donate electrons to Atom Y. Option A is the correct answer.
Atom X has 6 valence electrons, which means it has 6 electrons in its outermost energy level. Atom Y has 2 valence electrons, indicating that it has 2 electrons in its outermost energy level. In a chemical reaction, atoms tend to either donate or accept electrons to achieve a stable electron configuration. Option A is the correct answer.
Atom X has more valence electrons than Atom Y, suggesting that it is more likely to donate electrons. Since Atom Y has fewer valence electrons, it is more likely to accept electrons from Atom X. Therefore, based on the number of valence electrons, Atom X will donate electrons to Atom Y in order to achieve a stable electron configuration.
Learn more about Atom here:
https://brainly.com/question/17545314
#SPJ2

Given that the half-life (t1/2) of 226Ra is 1.6 x 103 years, calculate the decay constant (k)
for this isotope.
Answers
The decay constant (k) for the 226Ra isotope with a half-life of 1.6 x 10^3 years is approximately 4.33 x 10^-4 year^-1.
To calculate the decay constant (k) for the 226Ra isotope, we can use the formula: k = ln(2) / t1/2, where ln(2) represents the natural logarithm of 2 and t1/2 is the half-life.
In this case, the half-life of 226Ra is given as 1.6 x 10^3 years.
By substituting this value into the formula, we get: k = ln(2) / (1.6 x 10^3).
Evaluating this expression gives us a decay constant of approximately 4.33 x 10^-4 year^-1. This value indicates the rate at which the 226Ra isotope decays over time.
Learn more about decay constant here:
https://brainly.com/question/9712130
#SPJ11
From the values of Delta H and Delta S predict which of the following reactions would be spontaneous at 25degree C. Calculate the minimum temperature at which each reaction will become spontaneous. Enter "NONE" if the reaction is not spontaneous at any temperature. (a) Delta H = 12.6 kJ/mol, Delta S = 93 J/K middot mol spontaneous at 25degree C not spontaneous at 25degree C (b) Delta H = 9.5 kJ/mol, Delta S = -94.0 J/K middot mol spontaneous at 25degree C not spontaneous at 25degree C
Answers
(a) The reaction with Delta H = 12.6 kJ/mol and Delta S = 93 J/K·mol is spontaneous at 25°C.
(b) The reaction with Delta H = 9.5 kJ/mol and Delta S = -94.0 J/K·mol is not spontaneous at 25°C.
To determine whether a reaction is spontaneous at a given temperature, we can use the Gibbs free energy equation: Delta G = Delta H - T·Delta S, where Delta G is the change in Gibbs free energy, Delta H is the change in enthalpy, Delta S is the change in entropy, and T is the temperature in Kelvin.
For a reaction to be spontaneous, Delta G must be negative. If Delta H is negative (exothermic) and Delta S is positive (increase in disorder), the reaction is more likely to be spontaneous.
(a) For the reaction with Delta H = 12.6 kJ/mol and Delta S = 93 J/K·mol, we have Delta G = 12.6 kJ/mol - (25 + 273) K·(93 J/K·mol/1000 J/kJ) = -5.25 kJ/mol. Since Delta G is negative, the reaction is spontaneous at 25°C.
(b) For the reaction with Delta H = 9.5 kJ/mol and Delta S = -94.0 J/K·mol, we have Delta G = 9.5 kJ/mol - (25 + 273) K·(-94.0 J/K·mol/1000 J/kJ) = 3.57 kJ/mol. Since Delta G is positive, the reaction is not spontaneous at 25°C.
To determine the minimum temperature at which a non-spontaneous reaction becomes spontaneous, we can set Delta G equal to zero and solve for T in the equation Delta G = Delta H - T·Delta S. However, in this case, both reactions are either spontaneous or non-spontaneous at 25°C, so we do not need to calculate the minimum temperature.
To know more about Gibbs free energy refer here:
https://brainly.com/question/29753420#
#SPJ11
What volume of 0.200 M Na2CO3 (Mm = 106 g/mol) solution contains 53.0 g of Na2CO3?
Question 1 options:
0.200 L of solution
0.400 L of solution
0.500 L of solution
1.60 L of solution
2.50 L of solution
Answers
Answer:
volume in Liter = 2.50 L
Explanation:
Given:
Na2CO3 = 0.2 M
Molar mass of Na2CO3 = 106 g/mol
Mass of Na2CO3 solution = 53 gram
Find:
Volume of Na2CO3
Computation:
Number of mol of Na2CO3 = (53 g) / (1.06 x 10² g/mol)
Number of mol of Na2CO3 = 0.5 mol
M = Number of mol / volume in Liter
0.2 = 0.5/ volume in Liter
volume in Liter = 0.5 / 0.2
volume in Liter = 2.50 L
Why is only increasing the ph of the ocean not enough to restore the ocean’s equilibrium?.
Answers
The increasing pH of an ocean is not enough to restore the equilibrium as seawater becomes more acidic, and the pH of the water drops. This process binds carbonate ions, reducing their abundance.
What is the ocean's equilibrium?Equilibrium is a state where the presence of base and acid is in equal quantity.
Increased pH, leads to more acidity and reduces the number of ions.
Ions are necessary for corals, oysters, mussels, and many other shelled organisms that require building their shells and skeletons.
Thus, increasing pH of an ocean is not enough to restore the equilibrium as seawater becomes more acidic, and the pH of the water drops. This process binds carbonate ions, reducing their abundance.
Learn more about equilibrium
https://brainly.com/question/13463225
#SPJ1
What music group demonstrates how they use sound technology when recording songs?
a.) creed
b.) foo fighters
c.)pearl jam
d.)sound garden
Answers
The music group which demonstrates how they use sound technology when recording songs is foo fighters
Who are foo fighters?Foo Fighters are formed in Seattle, Washington. They are group of music band who sings rock music. The band was formed in 1994 by a drummer, Dave Grohl.
The band has won so many awards which includes best rock album.
Therefore, Foo fighters is a music group which demonstrates how they use sound technology when recording songs.
Learn more about music:
https://brainly.com/question/17523475
2. A force of 600 Newtons acts on a ball for 1.5 seconds after being hit by a volleyball player.
a. What is the change in momentum of the ball?
b. If the ball has a mass of 275 grams, what is its velocity?
Answers
a) Data:
F = 600 N
t = 1.5 s
The equation for Impulse:
Impulse = I = F x t,
then the change in momentum: pf - pi
Impulse and the change in momentum are related like this:
pf - pi = I = F x t = 600 N x 1.5 s = 900 N.s
Therefore the change in momentum: pf-pi = 900 N.s
b) Velocity (t) = initial velocity + a x t
We can assume initial velocity = 0 m/s
From F = m x a = mass x acceleration, we clear "a",
m = 275 g = 0.275 kg (1 kg = 1000 g)
Then, F/m = a => 600 N/0.275 kg = 2182 m/s^2
Velocity (t) = 0 + 2182 m/s^2 x 1.5 s = 3273 m/s
Velocity = 3273 m/s
SURELY SOMEONE HELP it’s urgent plllss I’ll brainlist u/5 star!!! answer the ones u know. :)
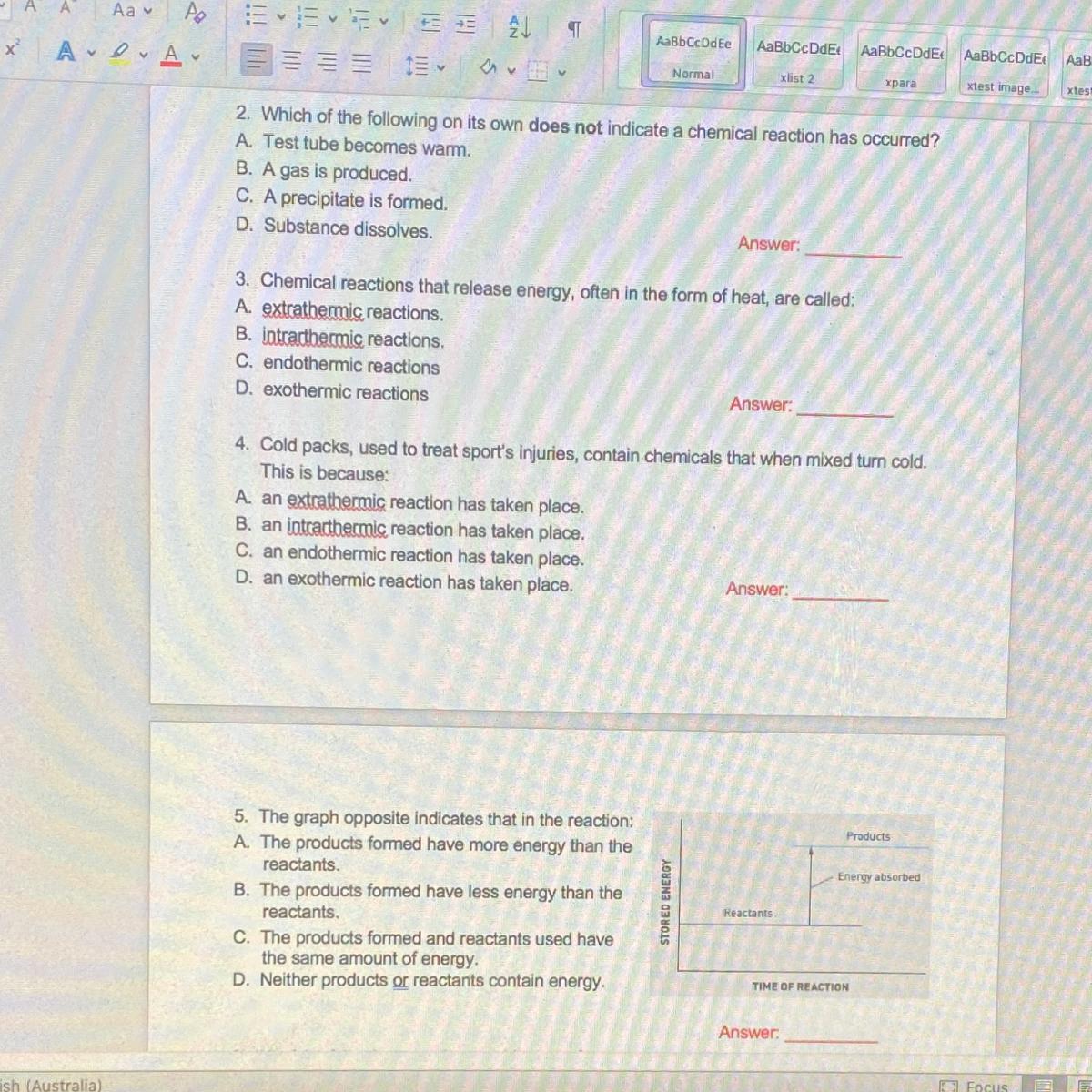
Answers
Answer:
3 exothermic reaction. only that much
Hi,
These are the answers.
• Question 2. C , Substance dissolves
• Question 3. D , Exothermic reaction
• Question 4. C , Endothermic reaction takes place
• Question 5. A , the products formed has more energy than reactants
Hope it helps you... pls mark brainliest if it helped you
how many molecules of lithium chloride are in 78.40 g? the molar mass of lithium chloride is 42.39 gmol.
Answers
Answer:
To find the number of molecules of lithium chloride in 78.40 g, we need to first convert the mass to moles using the molar mass of lithium chloride:
moles = mass / molar mass
moles = 78.40 g / 42.39 g/mol
moles = 1.849 mol
Now we can use Avogadro's number to convert from moles to molecules:
molecules = moles x Avogadro's number
molecules = 1.849 mol x 6.022 x 10^23 molecules/mol
molecules = 1.111 x 10^24 molecules
Therefore, there are 1.111 x 10^24 molecules of lithium chloride in 78.40 g.
Explanation:
How many atoms of Carbon C are in 3 molecules of Octane 3C8H18
Answers
Answer:
24 atoms
Explanation:3C8
3*8=24
28 atoms of carbons are there in 3 molecules of octane.
What is an atom?
A chemical element is uniquely defined by its atoms, which are tiny pieces of substance. A core nucleus surrounded by one or more negatively charged electrons makes up an atom.
Octane has an eight carbon atom straight chain alkane.
so, the three molecules of octane will have = 3 * 8 = 24 atoms.
Thus , 28 atoms of carbons are there in 3 molecules of octane.
To learn more about atoms, refer to the below link:
https://brainly.com/question/13933704
# SPJ2
What is the name of Se2Cl7?

Answers
Answer:
diselenium heptachloride
Explanation:
Se2Cl7
Se = selenium
Cl = chlorine
Don't forget the "-ide"
Diselenium heptachloride

If you dilute 18.0 mL of the stock solution to a final volume of 0.250 L , what will be the concentration of the diluted solution?
Answers
The concentration of the diluted solution is equal to 0.072 times the concentration of the stock solution.
To determine the concentration of the diluted solution, we need to use the equation:
C1V1 = C2V2
Where:
C1 = concentration of the stock solution
V1 = volume of the stock solution
C2 = concentration of the diluted solution
V2 = volume of the diluted solution
In this case, we have:
C1 = concentration of the stock solution (unknown)
V1 = 18.0 mL (milliliters)
C2 = concentration of the diluted solution (unknown)
V2 = 0.250 L (liters)
Since the units need to be consistent, we should convert the volume of the stock solution to liters:
V1 = 18.0 mL = 18.0 mL * (1 L / 1000 mL) = 0.018 L
Plugging the values into the equation, we have:
C1 * 0.018 L = C2 * 0.250 L
Now we can solve for C2, the concentration of the diluted solution:
C2 = (C1 * 0.018 L) / (0.250 L)
Simplifying the equation, we get:
C2 = 0.072 C1
This means that the concentration of the diluted solution is equal to 0.072 times the concentration of the stock solution.
In summary, to find the concentration of the diluted solution, you would multiply the concentration of the stock solution by 0.072. However, since the concentration of the stock solution is not provided in the question, we cannot calculate the exact concentration of the diluted solution.
Know more about Concentration here:
https://brainly.com/question/17206790
#SPJ8
define SI unit system
Answers
Answer:
The SI base units are the standard units of measurement defined by the International System of Units (SI) for the seven base quantities of what is now known as the International System of Quantities: they are notably a basic set from which all other SI units can be derived.
E. Mark each description as an exothermic or endothermic reaction. (2 points)
Description
Exothermic
Endothermic
A + heat → B
1
-ΔΗ
Energy diagram:
I
Energy of reactants
greater than energy of
products

Answers
Answer:
1. Endothermic
2. Exothermic
3. Exothermic
4. Endothermic
Explanation:
A P E X
If you could repeat the lab and make it better, what would you do differently and why?
There are always ways that labs can be improved. Now that you are a veteran of this lab and have experience with the procedure, offer some advice to the next scientist about what you suggest and why. Your answer should be at least two to three sentences in length.
need help with lab!- earth and space science 1
Answers
In order to obtain more accurate results as well as to improve the efficiency of the laboratory procedure, the following recommendations are given:
There should be accurate calibration of instrumentsThe samples should be properly labeledRepeated measurements should be takenHow can improvements be done to a lab to obtain better results?Improving laboratory results can be achieved through several strategies aimed at enhancing experimental conditions, equipment, procedures, and data analysis.
Some possible methods for improving laboratory performance:
Regularly calibrate and maintain laboratory instruments and equipment to ensure accuracy and reliability. Implement robust quality control measures by using appropriate standards, controls, and reference materials. Develop and follow standardized operating procedures for all experiments and tests.Proper labeling, preservation, and storage at appropriate temperatures.Learn more about lab procedures at: https://brainly.com/question/13517732
#SPJ1
A gas at 600 torr and 300 K is heated to a final volume of 1000 torr. What is the final temperature of the gas?
Answers
Answer:
500K
Explanation:
We use Charle's law that states that at a constant pressure the volume of a given mass of a gas varies linearly with absolute temperature of the gas.
V ∝ T
V = kT
∴ k is the proportionality constant
v/T = as it a ratio between volume and temperature the value of k would be a constant quantity.
Therefore it can be written as:
v1 / T1 = V2 / T2
Now solving the question:
V1 = 600 torr
T1 = 300 K
V2 = 1000 torr
T2 = ?
600/300 = 1000/T2
T2= (1000 x 300)/600
T2= 500 K