when a compound is dissolved in hot ethanol during a recrystallization, what is changing on a molecular level?
Answers
During a recrystallization, when a compound is dissolved in hot ethanol, the chemical bonds between the molecules of the compound are broken down due to the high temperature of the solvent.
This results in the separation of the constituent ions or molecules of the compound, which dissolve in the solvent to form a homogeneous solution. Upon cooling, the solution becomes supersaturated, and the constituent ions or molecules come together to form a pure crystalline solid.
Recrystallization is a common process used in chemistry to purify solid compounds. The process involves dissolving the impure compound in a solvent at a high temperature, followed by cooling the solution to allow the formation of pure crystals. In the case of using hot ethanol as a solvent, the high temperature causes the chemical bonds between the molecules of the compound to break down, resulting in ions or molecules separating from each other. This separation allows the constituent ions or molecules to be dissolved in the solvent, forming a homogeneous solution. Some compounds may require a co-solvent to dissolve completely.
Upon cooling, the solubility of the compound decreases, and the solution becomes supersaturated, which means that the amount of the compound dissolved in the solvent exceeds its normal solubility level. As a result, the constituent ions or molecules come together to form a pure crystalline solid, leaving behind any impurities or contaminants that may have been present in the original sample. This process of recrystallization is critical in obtaining pure chemicals, and the use of hot ethanol as a solvent can significantly enhance the efficiency of the process.
To learn more about recrystalization click brainly.com/question/15703840
#SPJ11
Related Questions
The _____
includes the top portion of Earth's crust, all the waters that cover
Earth's surface, and the surrounding atmosphere.
Answers
Answer:
biosphere
You can even double check on g00gle.
Hands moving on a battery-operated clock is an example of what kind of
energy conversion?
A. Heat energy being converted to gravitational potential energy
B. Gravitational potential energy being converted to heat energy
C. Chemical potential energy being converted to kinetic energy
D. Kinetic energy being converted to chemical potential energy
Answers
Answer:
Chemical potential energy being converted to kinetic energy
Explanation:
Write the general formula of an alkane and use this to predict the 97th member
of the alkane series.
Answers
Explanation:
The general formula of an alkane is given as;
CₙH₂ₙ₊₂
n is the number of the member;
For the 97th member in the series;
n = 97;
2n + 2 = 2(97) + 2 = 196
So, the 97th member is;
C₉₇H₁₉₆
This is how to apply the formula of alkanes which are saturated hydrocarbons to find any member of the series.
2. If you put 156. 32g barium hydroxide into this reaction, how much aluminium hydroxide can be
produced?
Answers
When 156.32 g of barium hydroxide is reacted, approximately 142.34 g of aluminum hydroxide can be produced, based on the balanced chemical equation and stoichiometry.
To determine the amount of aluminum hydroxide that can be produced when 156.32 g of barium hydroxide is reacted, we need to consider the balanced chemical equation for the reaction and use stoichiometry.
The balanced chemical equation for the reaction is:
Ba(OH)2 + 2AlCl3 → 2Al(OH)3 + 3BaCl2
From the balanced equation, we can see that for every 1 mole of Ba(OH)2, 2 moles of Al(OH)3 are produced.
First, we need to calculate the number of moles of barium hydroxide (Ba(OH)2) in 156.32 g:
Molar mass of Ba(OH)2 = (137.33 g/mol + 2(16.00 g/mol + 1.01 g/mol)) = 171.34 g/mol
Moles of Ba(OH)2 = mass / molar mass = 156.32 g / 171.34 g/mol = 0.911 mol
Now, using the stoichiometry of the balanced equation, we can determine the moles of aluminum hydroxide (Al(OH)3) produced:
Moles of Al(OH)3 = 2 × Moles of Ba(OH)2 = 2 × 0.911 mol = 1.822 mol
Finally, to convert the moles of aluminum hydroxide to grams, we need to multiply by the molar mass of Al(OH)3:
Molar mass of Al(OH)3 = (26.98 g/mol + 3(16.00 g/mol + 1.01 g/mol)) = 78.00 g/mol
Mass of Al(OH)3 = Moles of Al(OH)3 × molar mass = 1.822 mol × 78.00 g/mol = 142.34 g
Therefore, when 156.32 g of barium hydroxide is reacted, approximately 142.34 g of aluminum hydroxide can be produced.
For more such questions on barium hydroxide visit;
https://brainly.com/question/29344018
#SPJ8
Can someone help me with that question

Answers
Answer:
Gene - determines the trait (phenotype)
What pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4
can someone help me i need it before 10:10

Answers
Answer:
B 6NO A 5N2 C 6H2O D 4Nhd
Explanation:
Which of the following is not related to metallic bond?
A. Alloy
B. Shared electrons
C. Transferred electrons
D. Sea of electrons
Answers
Answer: B. Shared electrons
Explanation: Electrons are shared in covalent bonds. The other options are all properties of metallic bonds.
If I have 340 mL of a 1.5 M NaBr solution, what will the concentration be if I add 560 mL more water to it?
Answers
Answer:
0.5667 M ≅ 0.57 M.
Explanation:
It is known that the no. of millimoles of a solution before dilution is equal to the no. of millimoles of the solution after the dilution.
It can be expressed as:
(MV) before dilution = (MV) after dilution.
M before dilution = 1.5 M, V before dilution = 340 mL.
M after dilution = ??? M, V after dilution = 340 mL + 560 mL = 900 mL.
∴ M after dilution = (MV) before dilution/(V) after dilution = (1.5 M)(340 mL)/(900 mL) = 0.5667 M ≅ 0.57 M.
It took 3 hours for a train to travel the distance between two cities at a
velocity of 290 miles/hour. What is the DISTANCE between the two cities?
Pls hurry!
Answers
Answer:
The answer is 870 milesExplanation:
The distance covered by an object given it's velocity and time taken can be found by using the formula
distance = velocity × timeFrom the question
velocity = 290 miles/hour
time = 3 hours
The distance between the two cities is
distance = 290 × 3
We have the final answer as
870 milesHope this helps you
Which set correctly orders the atoms from HIGHEST to LOWEST ionization energy?

Answers
Answer:
Option D
Explanation:
Ionization energy increases left to right in a period and decreases top to bottom in a groups.
Ar is in Group 13
S is in Group 15
P is in Group 16
Al is in Group 18
They are all in the same period so decide by the group numbers if left is the highest (group 18) and right (group 13) is the lowest.
The order: Ar, S, P, Al
Hope this is clear. Good luck with chemistry! :)
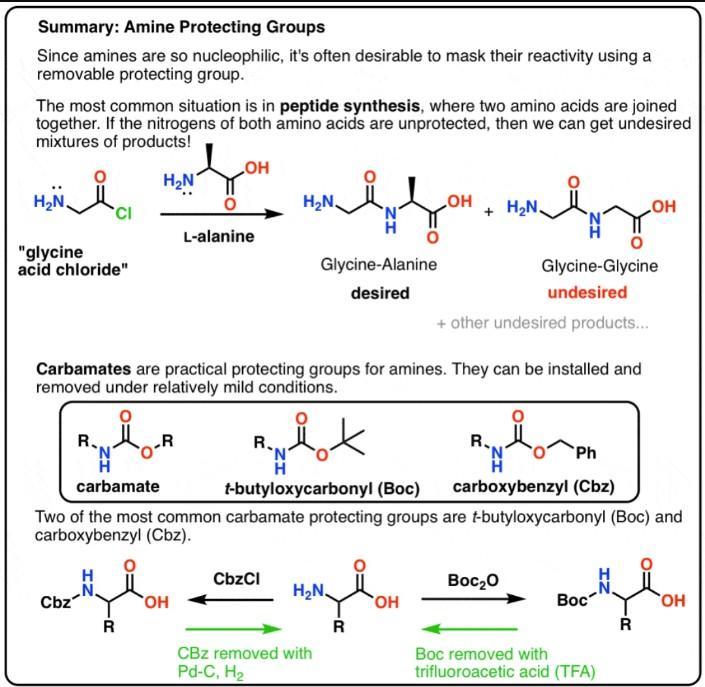
5.the acyl group is a protecting group for amines which can be deprotected by treatment with sodium hydroxide. please write mechanisms for both the protection and deprotection steps
Answers
Protecting Groups for Amines:
Amine protecting groups are essential for the synthesis of peptides.Carbamates are useful protecting groups for amines. They can be installed and removed under relatively mild conditions.One of the most common carbonate protecting groups is the t-butyloxycarbonyl (Boc) protecting group. It can be removed with strong acid (trifluoro acetic acid) or heat.The carboxybenzyl (CBz) group has a benzyl group and can be removed using catalytic hydrogenation (Pd-C, H₂)The fluorenyl methoxy (FMoc) group can be removed with an amine base (e.g. R₂NH)If multiple protecting groups are present on the same molecule, it's useful to choose them such that they can each be removed under different sets of conditions (e.g. acidic, basic, hydrogenation).Learn more about Protecting Groups for Amines are:
https://brainly.com/question/2575635
#SPJ4
Which type of chemistry studies the chemical reactions that occur in the human body?
Answers
Answer:
The type of Chemistry that studies the chemical reactions that occur in the human body, is Biochemistry.
Explanation:
That is true ofcourse so yes
The part of the water cycle where water from the ocean is changed into vapor is
driven by energy from the ?
A) lunar tides
B) earth's gravity
C) magnitisim
D) sun's heat
Answers
Answer:
D) Sun's heat
Explanation:
The water cycle is driven primarily by the energy from the sun. This solar energy drives the cycle by evaporating water from the oceans, lakes and rivers.
Answer:
D) sun's heat
Explanation:
The sun's heat provides enough energy to water molecules at the surface so they move fast enough to escape from the water. The water does not need to boil.
There is an experiment where a Gummy
Bear is sacrificed for the sake of science
. The 2nd part of the experiment involves
tossing a Gummy Bear into molten
potassium chlorate. As a result, the sugar
reacts with oxygen and generates purple sparks and a
lot of heat. Balance the reaction below so that the
Gummy Bear would not have died in vain.
Answers
an experiment where a Gummy Bear is sacrificed for the sake of science The 2nd part of the experiment involves tossing a Gummy Bear into molten potassium chlorate. As a result, the sucrose reacts with oxygen and generates purple sparks and a lot of heat and The balanced reaction looks like :
C₁₂H₂₂O₁₁ (s) + 8KClO₃ (s) = 12CO₂ (g) + 11H₂O (g) + 8KCl (s)
When the potassium chlorate is heated, it decomposes into potassium chloride and oxide, as seen below:
2KClO₃(s) = 2KCl(s) + 3O₂(g)
When the gummy bear is dropped, the oxide from the decomposition of potassium chlorate reacts with the glucose molecule in sucrose. This reaction is a spontaneous combustion reaction:
C₆H₁₂O₆ (s) + 6O₂(g) = 6CO₂(g) + 6H₂O (g)
The overall reaction is seen below:
C₁₂H₂₂O₁₁ (s) + 8KClO₃ (s) = 12CO₂ (g) + 11H₂O (g) + 8KCl (s)
Learn more about Sucrose, here:
https://brainly.com/question/29186350
#SPJ1
strong acids and bases dissolve completely in water. therefore, they are classified as electrolytes.
Answers
Strong acids and bases dissociate dissolve completely in water. therefore, they are classified as electrolytes.
What do the body's electrolytes do?
Your body contains electrolytes, which are minerals with an electric charge. They can be found in various bodily fluids such as urine, tissues, and blood.
Electrolytes are crucial because they: Maintain a healthy balance of bodily fluids. Achieve pH balance throughout your body. The important electrolytes are sodium, potassium, chloride, magnesium, calcium, phosphate, and bicarbonates.
What transpires if your body is deficient in electrolytes?
The activities of your body, including blood coagulation, muscle contractions, acid balance, and fluid control, might be hampered when your body's electrolyte levels drop.
Since your heart is a muscle, electrolytes aid in controlling your heartbeat.
Learn more about electrolytes
brainly.com/question/28699046
#SPJ4
A chemical equation must be balanced. this means that the same _____ and _____ of atoms must appear on both sides of the equation.
Answers
A chemical equation must be balanced. this means that the same number and type of atoms must appear on both sides of the equation .
In other words, both sides of the reaction have an equal balance of mass and charge. The amount and types of atoms on both sides of the reaction arrow must match for the chemical equation to be balanced.
To comply with the law of conservation of mass, which stipulates that matter cannot be generated or destroyed in a closed system, chemical equations must be balanced.
To learn about Chemical equation please click here,
https://brainly.com/question/28294176
#SPJ4
A substance with a *pH of 13* tells you that the substance is mildly basic or strongly basic?
Answers
Answer:
Strongly basic
Explanation:
pH is a measure of the acidity or basicity of a solution, with values ranging from 0 to 14. A pH of 7 is considered neutral, values below 7 indicate acidity, and values above 7 indicate basicity. As pH increases beyond 7, the basicity of the substance becomes stronger.
Use this pH Scale to further aid you in your studies of Acid Base Chem :)

an unknown liquid occupies a volume of 5ml and has a mass of 40 grams. Find its density
Answers
Answer:
8000 kilogram/cubic meter
Explanation:
I used an online calculator.
Hope I helped!
According to the big bang theory, the universe—all of the stars, planets, and galaxies everywhere—began as a very small, dense mass. This mass expanded suddenly in an incredibly hot explosion-like event, and it has spent the last 13.7 billion years rapidly expanding and cooling.
In 1964, which of the following space technologies provided scientists with the best evidence that the universe was created in the big bang?
A.
a lunar module
B.
a Mars rover
C.
a radio telescope
D.
a weather satellite
Answers
during metamorphism, what is the major effect of chemically active fluids? group of answer choices they prevent partial melting so solid rocks can undergo very high temperature regional metamorphism. they facilitate the formation of schistosity and gneissic banding in hornfels and slates. they increase the pressures in deeply buried, regional-metamorphic zones. they aid in the movement of dissolved silicate constituents and facilitate growth of the mineral grains.
Answers
Chemically active fluids primarily aid in the mobility of dissolved silicate components and facilitate the development of the mineral grains during metamorphism.
What are chemically active fluids' principal effects during metamorphism?During metamorphism, chemically active fluids that are present between the mineral grains help to promote ion movement and the re-crystallization of both old and new minerals.
What is the primary outcome of metamorphosis?Rocks that have undergone metamorphism include igneous, sedimentary, and other types of metamorphic rocks. The variations include the creation of new minerals, an increase in grain size, and adjustments to the structure or texture of the rock, depending on the chemical composition of the original rock and the degree of metamorphism.
To know more about metamorphism visit:-
https://brainly.com/question/7383699
#SPJ1
A nurse is preparing to administer multiple medications to a client who has an enteral feeding tube. Which of the following actions should the nurse plan to take?
Flush the tube with 15 mL of sterile water
* Each medication should be dissolved in at least 30 mL of warm sterile water
* Medications should be drawn up separately
* If the nurse encounters resistance when adm. meds, he should stop and contact the provider
Answers
When administering multiple medications to a client with an enteral feeding tube, the nurse should take several precautions to ensure the safe and effective delivery of the drugs.
Firstly, the nurse should flush the tube with 15 mL of sterile water before administering any medications to clear any residual feedings or other substances from the tube. Secondly, each medication should be dissolved in at least 30 mL of warm sterile water to prevent clogging and ensure that the medication is delivered properly. Thirdly, medications should be drawn up separately to avoid any potential interactions or incompatibilities between different drugs. Finally, if the nurse encounters resistance when administering medications, they should stop and contact the provider for further instructions. By following these guidelines, the nurse can help ensure that the client receives the full therapeutic benefit of each medication while minimizing the risk of adverse effects or complications.
To learn more about medications click here https://brainly.com/question/30453042
#SPJ11
The ph of a solution is 7. When acid is added to the solution, the hydronium ion concentration becomes 100 times greater. What is the ph of the new solution?.
Answers
After acid was added to the solution and the hydronium ion concentration becomes 100 times greater, the pH of the new solution is 5.
What is pH of solution?The pH of a solution is defined as the logarithm of the reciprocal of the hydrogen ion concentration [H+] of the given solution.
It is expressed as;
pH = -log[ H⁺]
Given that;
pH of the solution = 7Hydronium ion concentration H⁺ = ?pH = -log[ H⁺]
H⁺ = 10^( -pH )
H⁺ = 10^( -7 )
H⁺ = 10⁻⁷
When acid is added to the solution, the hydronium ion concentration becomes 100 times greater.
H⁺ = 10⁻⁷ × 100
H⁺ = 10⁻⁵
pH of the new solution will be;
pH = -log[ H⁺ ]
pH = -log[ 10⁻⁵ ]
pH = 5
Therefore, after acid was added to the solution and the hydronium ion concentration becomes 100 times greater, the pH of the new solution is 5.
Learn more about pH & pOH here: brainly.com/question/17144456
#SPJ1
Describe why hydrogen bonds form between water molecules
Answers
Answer:
Water molecules forming hydrogen bonds with one another. The partial negative charge on the O of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of other molecules. Water molecules are also attracted to other polar molecules and to ions.
Explanation:
When a positron and an electron annihilate one another, the resulting mass is completely converted to energy. Calculate the energy associated with this process in kJ/mol.
Answers
The energy associated with the annihilation of a positron and an electron is approximately 2.7416 × 10⁻⁸ kilojoules per mole.
When a positron and an electron annihilate each other, their combined mass is completely converted into energy according to Einstein's mass-energy equivalence principle (E=mc²). To calculate the energy associated with this process in kilojoules per mole (kJ/mol), we need to determine the mass of the positron-electron pair and then apply the appropriate conversion factors.
The mass of an electron is approximately 9.10938356 × 10⁻³¹ kilograms, and since the positron has the same mass, the combined mass of the pair is twice that value. To convert this mass to moles, we need to divide it by Avogadro's number, which is 6.022 × 10²³ particles/mol.
Now, to calculate the energy associated with the annihilation process, we can use the equation E=mc². The mass of the positron-electron pair is multiplied by the square of the speed of light, c, which is approximately 2.998 × 10⁸ meters per second.
Therefore, the energy associated with the annihilation of a positron and an electron can be calculated as follows:
Energy (E) = (2 * mass of electron) * (speed of light)²
Substituting the known values and performing the calculation, we find:
E = (2 * 9.10938356 × 10⁻³¹ kg) * (2.998 × 10⁸ m/s)²
Finally, convert the energy to kilojoules per mole by dividing by Avogadro's number:
Energy (E) = [(2 * 9.10938356 × 10⁻³¹ kg) * (2.998 × 10⁸ m/s)²] / (6.022 × 10²³ particles/mol)
= 2.7416 × 10⁻³⁶ kg m²/s²/particles * mol
To convert this energy to kilojoules per mole, we need to multiply by the conversion factor. The conversion factor is given by 1 J/mol = 1 kg m²/s²/mol. Additionally, 1 kJ = 1000 J.
Energy (E) = (2.7416 × 10⁻³⁶ kg m²/s²/particles * mol) * (1 J/mol / 1 kg m²/s²) * (1 kJ / 1000 J)
= 2.7416 × 10⁻³⁶ * 10⁻³ * 10⁻³ kJ/mol
= 2.7416 × 10⁻⁸ kJ/mol
Therefore, the energy associated with the annihilation of a positron and an electron is approximately 2.7416 × 10⁻⁸ kilojoules per mole.
Know more about Energy here:
https://brainly.com/question/1932868
#SPJ11
what volume of dinitrogen pentoxide gas can be synthesized from 2.54 l of nitrogen gas and 1.83 l of oxygen gas? (hint: it is a limiting reactant problem.)
Answers
The volume of dinitrogen pentoxide gas that can be synthesized is 5.08 liters.
To determine the volume of dinitrogen pentoxide (N2O5) gas that can be synthesized, we need to use the balanced chemical equation for the reaction between nitrogen gas (N2) and oxygen gas (O2) to form dinitrogen pentoxide.
The balanced equation is:
N2(g) + 2O2(g) → 2N2O5(g)
From the equation, we can see that the mole ratio between nitrogen gas and dinitrogen pentoxide is 1:2. This means that for every 1 mole of nitrogen gas, we will produce 2 moles of dinitrogen pentoxide.
First, we need to calculate the number of moles of nitrogen gas and oxygen gas present:
Moles of nitrogen gas = volume of nitrogen gas / molar volume of nitrogen gas at STP
Moles of nitrogen gas = 2.54 L / 22.4 L/mol = 0.1134 moles
Moles of oxygen gas = volume of oxygen gas / molar volume of oxygen gas at STP
Moles of oxygen gas = 1.83 L / 22.4 L/mol = 0.0817 moles
Since the mole ratio between nitrogen gas and dinitrogen pentoxide is 1:2, the limiting reactant is nitrogen gas because it has fewer moles.
According to the balanced equation, 1 mole of nitrogen gas produces 2 moles of dinitrogen pentoxide. Therefore, the number of moles of dinitrogen pentoxide produced is 2 times the number of moles of nitrogen gas:
Moles of dinitrogen pentoxide = 2 × moles of nitrogen gas
Moles of dinitrogen pentoxide = 2 × 0.1134 moles = 0.2268 moles
Finally, we can calculate the volume of dinitrogen pentoxide gas using the molar volume at STP:
Volume of dinitrogen pentoxide gas = moles of dinitrogen pentoxide × molar volume of dinitrogen pentoxide gas at STP
Volume of dinitrogen pentoxide gas = 0.2268 moles × 22.4 L/mol = 5.08 L
Know more about dinitrogen pentoxide gas here:
https://brainly.com/question/14008564
#SPJ11
How many atoms are in 1 mole of Carbon tetrachloride?
Answers
Answer:
There is one carbon atom in every carbon tetrachloride molecule, which means that there is an equal number of carbon atoms to carbon tetrachloride molecules. 5.089 x 1022 atoms of C.
Explanation:
Hope this helped, Have a Wonderful Day!!
Find w, x, y and z such that the following chemical reaction is balanced. w Ba3 N₂ + xH₂O →yBa(OH)2 + ZNH3
Answers
The values of balanced chemical reaction is w = 1, x = 6, y = 3, and z = 2
To balance the chemical equation:
1. Balancing nitrogen (N):
There are three nitrogen atoms on the left side (Ba₃N₂), so we need to place a coefficient of 3 in front of NH₃:
w Ba₃N₂ + x H₂O → y Ba(OH)₂ + 3 z NH₃
2. Balancing hydrogen (H):
There are six hydrogen atoms on the left side (2 × 3), so we need to place a coefficient of 6 in front of H₂O:
w Ba₃N₂ + 6 H₂O → y Ba(OH)₂ + 3 z NH₃
3. Balancing barium (Ba):
There are three barium atoms on the left side (3 × Ba₃N₂), so we need to place a coefficient of 3 in front of Ba(OH)₂:
w Ba₃N₂ + 6 H₂O → 3 y Ba(OH)₂ + 3 z NH₃
4. Balancing oxygen (O):
There are six oxygen atoms on the right side (6 × OH), so we need to place a coefficient of 3 in front of Ba(OH)₂:
w Ba₃N₂ + 6 H₂O → 3 Ba(OH)₂ + 3 z NH₃
Now the equation is balanced with the following coefficients:
w Ba₃N₂ + 6 H₂O → 3 Ba(OH)₂ + 3 z NH₃
Therefore, w = 1, x = 6, y = 3, and z = 2 would satisfy the balanced chemical equation.
Learn more about balanced chemical reactions at https://brainly.com/question/26694427
#SPJ11
Calculate the volume in mL of 53.2 g of .251M solution of HCl.
Show work please
Answers
Answer:
5.813 mL
Explanation:
You need to use the formula : vol = mass / molar mass x molarity
the molar mass of HCl is 36.46 and the problem gives you the rest of the equation
0.251M x 36.46 = 9.151
53.2g / 9.151 = 5.813 mL
*I don't know some of the units so that's why some of them are blank sorry
*I'm also sorry if this is wrong but it's what I did so..
What is the difference between a theory and a law in science