Which of the following comes first in the name of an ionic compound?(1 point) the number of ions present the number of ions present the negatively charged ion the negatively charged ion the neutral ion the neutral ion the positively charged ion
Answers
Answer:
The positively charged ion
Explanation:
Related Questions
If you could travel to any TIME PERIOD, when would go and why.
Answers
Answer:
I Would Go Back To When There Were Cavemen Because I Would Love To See How Things Went On And How Women/Men Handled Themselves
Explanation:
Why Would I Travel To When Cavemen Were Here?
Because I Have A Fuji Film And I Would Love To Come Back To This Day And Show Everyone That Cavemen/Dinosoars ARE Real
QUICKLY PLEASE: What is true about 1. 0 mol Ca and 1. 0 mol Mg? (3 points)
Answers
Both 1.0 mol of calcium (Ca) and 1.0 mol of magnesium (Mg) contain the same number of atoms (Avogadro's number, 6.022 x 10²³ atoms), but they differ in mass and chemical properties.
In order to compare 1.0 mol Ca and 1.0 mol Mg, we must first understand the concept of a mole. A mole is a unit of measurement that represents 6.022 x 10²³ particles (atoms, molecules, ions, etc.). This number, known as Avogadro's number, allows us to compare amounts of different substances.
Although 1.0 mol Ca and 1.0 mol Mg both contain the same number of atoms, their masses are different. The molar mass of Ca is 40.08 g/mol, while the molar mass of Mg is 24.31 g/mol.
Therefore, 1.0 mol Ca has a mass of 40.08 g, and 1.0 mol Mg has a mass of 24.31 g. Additionally, Ca and Mg are both alkaline earth metals but possess different chemical properties, such as reactivity and electron configurations.
To know more about Avogadro's number click on below link:
https://brainly.com/question/28812626#
#SPJ11
Scientists use bacteria to make medicines,
o True
o False
Answers
Answer:
true they do I yes they do
The shape of the directly sunlit portion of the Moon as viewed from
Earth
Answers
your answer:
lunar phases
Thorium-234 undergoes beta decay to form a daughter nuclide and a beta particle. What are the mass number and atomic number for the daughter nuclide?
A.230, 88
B.234, 89
C.230, 91
D. 234, 91
Answers
The mass number of the daughter nuclide will remain the same as Thorium-234 (mass number 234). The correct answer is D. 234, 91.
When there are too many protons or neutrons in a nucleus, one of the protons or neutrons will turn into the other, which is known as beta decay. During beta minus decay, a neutron transforms into a proton, electron, and antineutrino.
A daughter nuclide is created when a neutron in the nucleus decays into a proton during beta decay. While the mass number stays constant, the atomic number rises by 1.
We know the daughter nuclide will have an atomic number one unit higher than thorium (atomic number 90) because thorium-234 (Th-234) undergoes beta decay. The daughter nuclide will continue to have the same mass number as thorium-234 (mass number 234).
To know more about beta decay:
https://brainly.com/question/4184205
#SPJ4
A new element, Thorsonium, has two isotopes. Thorsonium isotopes have a mass of 255 amu and 257 amu. Given the average atomic mass of Thorsonium is 255.65 amu, calculate the percent abundance for each Thorsonium isotope.
Answers
Answer:
255 amu has relative abundance 67.5% and 257 has relative abundance 32.5%
Explanation:
Average atomic mass =sum of product of atomic mass and relative abundance all divided by hundred or total abundance
a) Water has a molar mass of 18 g/mol and a density of 1000 kg/m3 (or 1 g/cm3). Based on this data, estimate the number of water molecules per unit surface area of water.
b) The coordination number of water (i.e., the average number of "neighbors" each water molecule has) in the liquid state is 4. Neighboring water molecules attract each other via hydrogen bonds, each of which has a binding energy of roughly 10–20 J (although this number depends relatively strongly on temperature). Use this information to estimate the surface tension of water. How does your estimate compare to the observed figure (
γ water = 0.072 N/m ) (hints: Keep in mind that we can think of
surface tension as surface energy per unit area, and consider the energy needed to bring a molecule from the bulk to the surface)?
Answers
The estimate is still useful because it provides insight into the behavior of water molecules at the surface of the liquid.
Molar mass of water, M = 18g/molDensity of water, ρ = 1g/cm³ = 1000kg/m³The number of molecules per unit surface area of water can be estimated as follows:Number of water molecules per unit volume of water = Avogadro's number, NA / MNumber of water molecules per unit volume of water = 6.022 × 10²³ / 18 = 3.345 × 10²² / molThe number of molecules per unit surface area of water = the number of molecules per unit volume of water × the thickness of the water layer on the surface= 3.345 × 10²² / m³ × 1 × 10⁻⁸ m= 3.345 × 10¹⁴ / m²b)Given:Coordination number of water, CN = 4Binding energy of hydrogen bond, E = 10⁻²⁰ JThe surface tension of water, γ water = 0.072 N/mEnergy required to bring one molecule from the bulk of the liquid to the surface of the liquid, ΔE= γ water × AThe total binding energy of a water molecule in the liquid state = the binding energy of one hydrogen bond × the coordination number= 10⁻²⁰ J/bond × 4 bonds = 4 × 10⁻²⁰ JThe number of molecules per unit surface area of water = the energy required to bring one molecule from the bulk of the liquid to the surface of the liquid / the total binding energy of a water molecule in the liquid state= ΔE / 4 × 10⁻²⁰= 0.072 / (4 × 10⁻²⁰)= 1.8 × 10²⁰The surface tension of water can also be expressed as follows:γ water = (N / A) × EThe number of hydrogen bonds per unit area, N / A = γ water / E= 0.072 / 10⁻²⁰ = 7.2 × 10¹⁸ / m²The difference between the estimated value and the observed value is relatively large (about a factor of 25). It is because this is just an estimate, and it does not consider all the factors affecting the surface tension of water. However, the estimate is still useful because it provides insight into the behavior of water molecules at the surface of the liquid.
Learn more about liquid here:
https://brainly.com/question/20922015
#SPJ11
How are chemical and mechanical weathering the same?
Answers
Answer:
They break down materials into smaller pieces
Explanation:
Although using different methods
what is 0.1 n hcl standardization?
Answers
The abbreviation 0.1 N HCl means normal hydrochloric acid.By comparing a solution to a standard solution with a known concentration, standardisation is the process of determining the precise concentration
By comparing a solution to a standard solution with a known concentration, standardisation is the process of determining the precise concentration of the solution. As precise concentration measurements are necessary for many scientific and industrial applications, it is a crucial stage in chemical analysis. In order to determine the concentration of the unknown solution, the standardisation procedure entails mixing a measured amount of the standard solution with a known quantity of the solution being tested. The primary standard, which is a pure material with a known and steady concentration, is often the standard solution. Standardization is used in many disciplines, including chemistry, biology, and engineering, to assure
Learn more about standardisation here:
https://brainly.com/question/30457109
#SPJ4
RODINNIS
COURSES
onal Science
Attempt 1 of 2
Which of the following distinctions are used to identify sedimentary rock? Select all that apply.
o where is was formed
conditions it was formed under
n when it was formed
what it is composed of
how many layers it consists of
NEED HELP ASAP (check the picture)

Answers
where it was formed and. conditions it was formed under
If 0.08 moles of Fe are
reacted, how many moles of
H, are formed?
Answers
Based on the balanced equation, if we have 0.08 moles of Iron (Fe), we can conclude that 0.08 moles of Hydrogen (H₂) will be formed as well.
How to Calculate Mole in a Chemical EquationFirst we need to balance the chemical equation for the reaction involving Iron (Fe) and Hydrogen (H).
The reaction will be between Fe and HCl (hydrochloric acid) to produce hydrogen gas (H₂) and iron chloride (FeCl₂):
Fe + 2HCl → FeCl₂ + H₂
From the balanced equation, we can see that for every 1 mole of Fe reacted, 1 mole of H₂ is formed. Therefore, if we have 0.08 moles of Fe, we can conclude that 0.08 moles of H₂ will be formed as well.
Learn more about moles here:
https://brainly.com/question/15356425
#SPJ1
Answer the four questions to figure out the four digit code


Answers
Answer:
question 1 =c
question 2 =a
question 3 =d
question 4 =b
Why is the alcohol of a β-hydroxy ketone easier to eliminate than a normal alcohol?'
Answers
The alcohol of a β-hydroxy ketone is easier to eliminate compared to normal alcohol due to the presence of the β-carbon.
The β-carbon is adjacent to the carbonyl group (C=O) and forms a more stable transition state during elimination reactions. This enhanced stability is attributed to the conjugation of the β-carbon with the carbonyl group, which delocalizes the electrons and lowers the energy barrier for elimination. As a result, the β-hydroxy ketone can undergo elimination reactions more readily, leading to the formation of a double bond and the removal of the alcohol group.
In β-hydroxy ketones, the β-carbon is directly connected to the carbon of the carbonyl group. The presence of this β-carbon provides an opportunity for resonance stabilization, where the electrons can delocalize between the oxygen of the hydroxyl group and the adjacent carbonyl carbon. This conjugation creates a more stable transition state during elimination reactions.
During elimination, the hydroxyl group on the β-carbon is protonated to form a good leaving group (water). The electrons from the C-O bond move toward the oxygen, creating a double bond between the β-carbon and the carbonyl carbon. This process is facilitated by the resonance stabilization mentioned earlier.
In contrast, normal alcohols lack the conjugation provided by the adjacent carbonyl group. Consequently, their elimination reactions require higher energy and are less favorable compared to β-hydroxy ketones.
Learn more about resonance here:
brainly.com/question/31039280
#SPJ11
What was the first brand of soda to be sold in an all-aluminum can?.
Answers
In 1959, Coors became the first brand of soda to introduce an all-aluminum can,
Revolutionizing the beverage industry. Coors introduced a two-piece aluminum can, which quickly became the standard for packaging beverages.
The adoption of aluminum cans brought several benefits over traditional glass bottles.
One advantage of aluminum cans is their lightweight nature, making them easier to handle and transport.
Additionally, aluminum cans are highly resistant to breakage, reducing the risk of damage during handling and distribution.
The use of aluminum cans also ensures an airtight seal, preserving the freshness and carbonation of the soda.
Coors' pioneering efforts in producing aluminum cans on a commercial scale paved the way for other soda brands to follow suit.
The switch from glass bottles to aluminum cans was driven by the cost-effectiveness of aluminum production, making it a more economical choice for beverage companies.
Overall, Coors' introduction of the two-piece aluminum can in 1959 marked a significant milestone in the beverage industry.
The lightweight, durable, and cost-effective nature of aluminum cans made them a preferred choice for packaging soda and paved the way for the widespread adoption of aluminum cans by other brands.
To know more about Aluminum Can here: https://brainly.com/question/32814093
#SPJ11
A clean, dry, and open empty flask is being heated in a water bath. Under which conditions before or after heating does the flask contain more gas molecules?
Answers
Before heating, the flask contains fewer gas molecules than after heating. When the flask is heated in the water bath, the temperature of the flask increases, causing the gas molecules inside to expand and take up more space.
The increased temperature also causes the gas molecules to move faster and collide more frequently, leading to an increase in pressure and the number of gas molecules in the flask.
As the temperature continues to rise, the air inside the flask becomes hotter and its pressure continues to increase.
At a certain point, the air inside the flask may reach a temperature that is high enough for some of the gas molecules to dissociate into individual atoms. This increases the number of particles in the flask, which leads to an even greater increase in pressure.
To read more about temperature, Visit-
https://brainly.com/question/7510619
#SPJ11
The molar enthalpy of vaporization of carbon disulfide is 26.74 kJ/mol, and its normal boiling point is 46C.
What is the vapor pressure (in torr) of CS2 at 0C?
Answers
The vapor pressure of carbon disulfide (CS₂) at 0°C is approximately 0.178 torr.
To determine the vapor pressure of carbon disulfide (CS₂) at 0°C, we can use the Clausius-Clapeyron equation:
ln(P₂/P₁) = -(ΔHᵥᵃᵖ/R) * (1/T₂ - 1/T₁)
Where:
P₁ = vapor pressure at temperature T₁ (known)
P₂ = vapor pressure at temperature T₂ (unknown)
ΔHᵥᵃᵖ = molar enthalpy of vaporization
R = gas constant (8.314 J/(mol·K))
T₁ = initial temperature (known)
T₂ = final temperature (unknown)
Given:
ΔHᵥᵃᵖ = 26.74 kJ/mol (convert to J/mol: 26.74 kJ/mol * 1000 J/1 kJ = 26740 J/mol)
T₁ = 46°C (convert to Kelvin: 46°C + 273.15 K = 319.15 K)
T₂ = 0°C (convert to Kelvin: 0°C + 273.15 K = 273.15 K)
R = 8.314 J/(mol·K)
Substituting the known values into the equation:
ln(P₂/P₁) = -(26740 J/mol / 8.314 J/(mol·K)) * (1/273.15 K - 1/319.15 K)
Simplifying the equation:
ln(P₂/P₁) = -3216.11 * (-0.003663 + 0.003128)
ln(P₂/P₁) = -3216.11 * 0.000535
ln(P₂/P₁) ≈ -1.723
To solve for P₂/P₁, we can take the exponential of both sides:
P₂/P₁ = e^(-1.723)
Using a calculator, e^(-1.723) ≈ 0.178
Finally, to find P₂ (vapor pressure at 0°C), we multiply P₁ by P₂/P₁:
P₂ = P₁ * (P₂/P₁)
P₂ = 1 torr * 0.178
P₂ ≈ 0.178 torr
Therefore, the vapor pressure of carbon disulfide (CS₂) at 0°C is approximately 0.178 torr.
Learn more about Vapor pressure from the link given below.
https://brainly.com/question/29640321
#SPJ4
Tell me some Viva questions for Experiment,Identify sign if chemical reaction
Answers
Some signs that indicate a chemical reaction has occurred include the production of gas, formation of a precipitate, a change in color or temperature, and the emission of light or sound.
There are several signs that indicate a chemical reaction has occurred. These include the formation of a gas, the formation of a precipitate, a color change, temperature change, and the evolution or absorption of heat. A gas can be produced when a solid reacts with an acid, resulting in the formation of carbon dioxide.
A precipitate may form when two aqueous solutions are mixed, resulting in the formation of an insoluble solid. A color change can occur when a substance undergoes a chemical reaction, resulting in the formation of a new substance with a different color. A temperature change may occur due to the release or absorption of heat during a chemical reaction.
To know more about reaction, here
brainly.com/question/29471566
#SPJ4
--The complete question is, What are some signs that indicate a chemical reaction has occurred?--
If you added 4 vials of 2.5 mg/0.5mL Albuterol solution to your nebulizer, how much is the total dosage of the Tx? How much saline would have to be added to achieve a continuous Tx lasting 3 hours using a nebulizer with an output of 12 mL/hr.
Answers
Answer:you would need to add 36 mL of saline to achieve a continuous treatment lasting 3 hours using a nebulizer with an output of 12 mL/hr.
Explanation:
To calculate the total dosage of Albuterol solution, we need to multiply the concentration of the solution (2.5 mg/0.5 mL) by the total volume of the solution used (4 vials, assuming each vial is 0.5 mL):
Total dosage of Albuterol = (2.5 mg/0.5 mL) * (0.5 mL/vial) * 4 vials
Total dosage of Albuterol = 20 mg
Therefore, the total dosage of Albuterol solution is 20 mg.
To calculate the amount of saline that needs to be added for a continuous treatment lasting 3 hours, we can use the nebulizer's output rate of 12 mL/hr:
Amount of saline needed = Nebulizer output rate * Treatment duration
Amount of saline needed = 12 mL/hr * 3 hr
Amount of saline needed = 36 mL
To achieve a continuous treatment lasting 3 hours using the nebulizer with an output of 12 mL/hr, an additional 34 mL of saline solution would need to be added.
If each vial of Albuterol solution contains 2.5 mg in 0.5 mL, then adding 4 vials would result in a total dosage of 10 mg (2.5 mg/vial * 4 vials).
To achieve a continuous treatment lasting 3 hours using a nebulizer with an output of 12 mL/hr, we need to calculate the amount of saline solution that needs to be added.
The nebulizer has an output of 12 mL/hr, so over 3 hours, it would deliver a total volume of 12 mL/hr * 3 hrs = 36 mL.
Since we have already added the 4 vials of Albuterol solution, we subtract that volume from the total desired volume of 36 mL to determine how much saline needs to be added.
Therefore, the amount of saline to be added would be 36 mL - 2 mL (4 vials * 0.5 mL/vial) = 34 mL.
Know more about Nebulizer here:
https://brainly.com/question/31455646
#SPJ11
A student bought a 1.55-ounce chocolate bar and left it in a car on a hot day.
How many ounces of chocolate are in the melted bar?

A.
Exactly 1.55 ounces

B.
An unknown number of ounces

C.
At least 1.55 ounces

D.
Less than 1.55 ounces
Answers
Answer:
A. Exactly 1.55 ounces
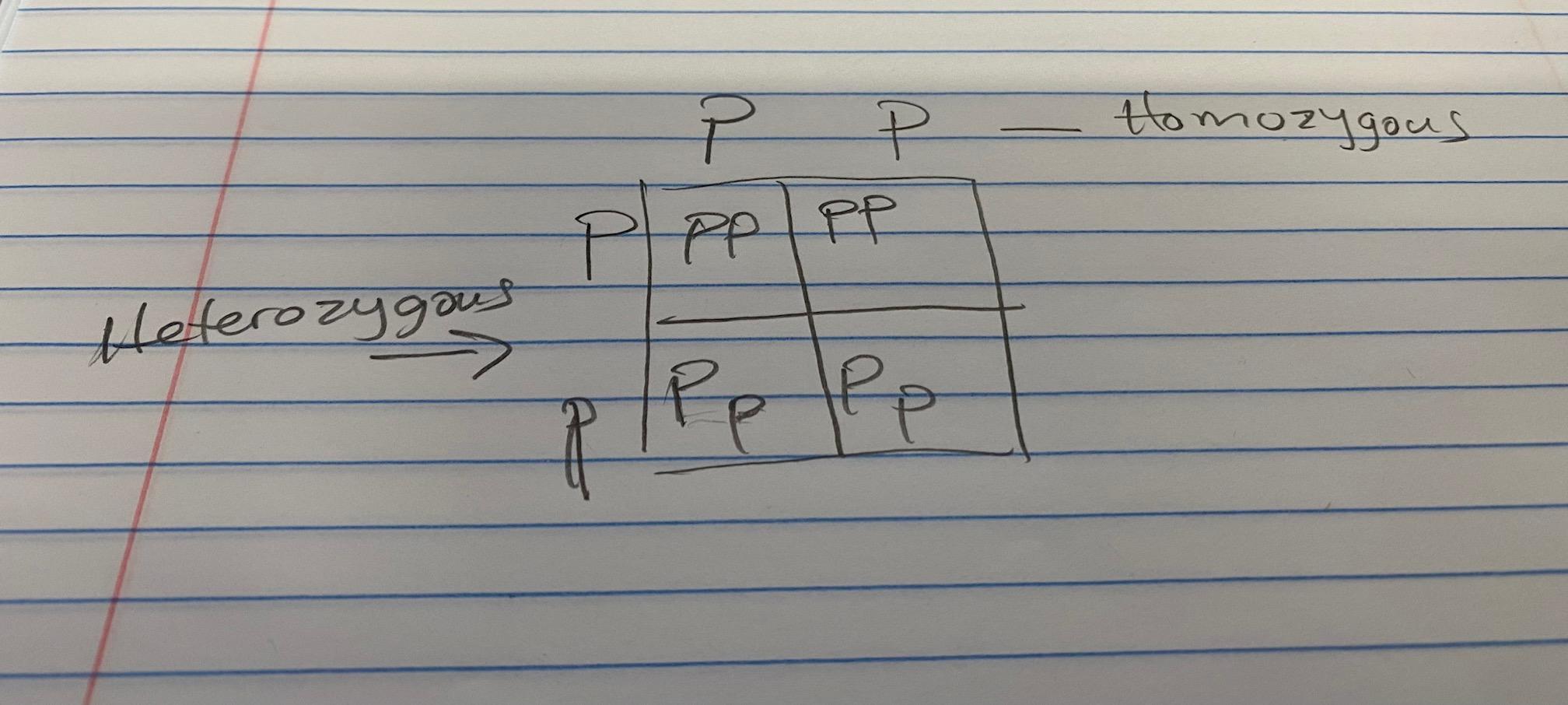
This is the picture btw because it wont let me say all the words.

Answers
Answer:
see image
D is the answer
Explanation:
see image
the box is like a mini multiplication table
what are the relative energy levels of the three staggered conformations of 2,3-dimethylbutane when looking down
Answers
Therefore, the relative energy levels of the three staggered conformations of 2,3-dimethylbutane, when looking down the carbon-carbon bond axis, are:
Anti-periplanar (lowest energy) < Gauche < Eclipsed (highest energy)
When looking down the carbon-carbon bond axis in 2,3-dimethylbutane, the three staggered conformations are:
Anti-periplanar (lowest energy): In this conformation, the two methyl groups are in a staggered arrangement, with one methyl group pointing up and the other pointing down. This conformation has the lowest energy due to the maximum separation between the bulky methyl groups.
Gauche: In this conformation, the two methyl groups are slightly closer to each other, resulting in some steric hindrance. One methyl group is pointing up, while the other is pointing to the side. The energy of the gauche conformation is slightly higher than the anti-periplanar conformation.
Eclipsed (highest energy): In this conformation, the two methyl groups are eclipsed, meaning they are closest to each other. Both methyl groups are pointing to the side. This conformation has the highest energy due to the significant steric hindrance between the bulky methyl groups.
Learn more about carbon bond here
https://brainly.com/question/29663260
#SPJ11
calculate the number of molecules of acetyl-scoa derived from a saturated fatty acid with 18 carbon atoms.
Answers
The beta-oxidation of an 18-carbon saturated fatty acid generates 9 acetyl-CoA molecules. This process is essential for energy production, as acetyl-CoA can be further metabolized in the citric acid cycle, also known as the Krebs cycle, to produce ATP.
To calculate the number of molecules of acetyl-CoA derived from a saturated fatty acid with 18 carbon atoms, we need to understand the biochemical process of fatty acid oxidation, also known as beta-oxidation. In this process, the fatty acid is broken down into two-carbon units, which form acetyl-CoA molecules.
Step 1: Determine the number of carbon atoms in the fatty acid.
The given saturated fatty acid has 18 carbon atoms.
Step 2: Determine the number of two-carbon units.
Since each acetyl-CoA molecule consists of two carbon atoms, we can find the number of two-carbon units by dividing the total number of carbon atoms by 2:
18 carbon atoms / 2 = 9 two-carbon units.
Step 3: Calculate the number of acetyl-CoA molecules.
As each two-carbon unit forms one acetyl-CoA molecule, the number of acetyl-CoA molecules derived from the 18-carbon saturated fatty acid is equal to the number of two-carbon units. Therefore, there are 9 acetyl-CoA molecules derived from this fatty acid.
To learn more about beta-oxidation, refer:-
https://brainly.com/question/29458295
#SPJ11
Drag and drop the words that accurately complete the chart below. Example a lion and a cheetah mistletoe on a tree a coyote eating a rabbit a remora and a shark clownfish and anemone parasitism friendship competition Type of Symbiosis mutualism 1:10 predation relationship commensalism collaboration alliance

Answers
Answer:
Lion and cheetah - Competition
Mistletoe on a tree - Parasitism
Coyote eating rabbit- Predatation
Remora and Shark - Mutualism
Clownfish and Anemone - Relationship
Explanation:
Describe the many different forms of energy involved with stretching and releasing a rubber band. What other processes are similar to this model?
Answers
Answer:
Conversion of kinetic energy to potential energy (chemo mechanical energy)
In the state of rest, the rubber is a tangled mass of long chained cross-linked polymer that due to their disorderliness are in a state of increased entropy. By pulling on the polymer, the applied kinetic energy stretches the polymer into straight chains, giving them order and reducing their entropy. The stretched rubber then has energy stored in the form of chemo mechanical energy which is a form of potential energy
Conversion of the stored potential energy in the stretched to kinetic energy
By remaining in a stretched condition, the rubber is in a state of high potential energy, when the force holding the rubber in place is removed, due to the laws of thermodynamics, the polymers in the rubber curls back to their state of "random" tangled mass releasing the stored potential energy in the process and doing work such as moving items placed in the rubber's path of motion such as an object that has weight, w then takes up the kinetic energy 1/2×m×v² which can can result in the flight of the object.
Explanation:
Answer:
Elastic potential energy------> kinetic energy --------> elastic potential energy
Explanation:
Elastic potential energy is the energy stored in a spiral spring. When a rubber band is stretched, this elastic potential energy is capable of doing work. This energy is just stored inside the rubber band and released when work is done by the rubber band.
Once the rubber band is released and it is in motion, the energy of the rubber is kinetic energy.
The system is similar to a simple pendulum.
A flexible container holding 1.25 moles of a gas expands from 25.0 L to 10.0 L when additional gas is added. How many moles of gas are in the expanded container?
Assume the tempurature and pressure are constant.

Answers
The number of moles of gas in the expanded container would be 0.5 moles.
Number of moles of gasesAccording to Avogadro's law, an equal volume of gases at the same temperature and pressure will contain the same number of molecules.
Avogadro's law can be mathematically expressed as:
\(v_1/n_1 = v_2/n_2\)
Where \(v_1\) = initial volume, \(v_2\) = final volume, \(n_1\) = the initial number of moles, and \(n_2\) = the final number of moles.
In this case, \(n_1\) = 1.25 moles, \(v_1\) = 25.0 L, \(v_2\) = 10.0 L
Thus, we are to make \(n_2\) the subject of the formula:
\(n_2 = v_2n_1/v_1\)
= 10 x 1.25/25
= 0.5 moles
In other words, the number of moles of gas in the expanded container is 0.5 moles.
More on Avogadro's law can be found here: https://brainly.com/question/4133756
#SPJ1
Answer: 13.00
Explanation: got it right
A scientific theory is
A. a general explanation which is highly probable and well supported by testing
B. a fact
C. the same as a scientific law
D. a vague idea
I did pick a random subject.
Answers
Chemistry help needed fast, please !
Standard Pressures: 1 atm = 760 torr = 760 mmH = 101.3 kPa = 101,300 Pa = 14.7 psi

Answers
Understanding the standard pressures and their conversion factors is essential to performing accurate measurements and calculations involving pressure.
Standard pressures are the reference points used to measure the pressure of a gas, which is a critical parameter to evaluate and understand various physical, chemical, and biological phenomena. There are several units of pressure measurement, including atm, torr, mmHg, kPa, Pa, and psi. However, they all can be converted to each other based on their relationship with the standard pressure of 1 atm.The standard pressure of 1 atm, which stands for atmosphere, is equivalent to 760 torr, 760 mmHg, 101.3 kPa, 101,300 Pa, or 14.7 psi. The standard pressure of 1 atm is the typical air pressure at sea level, where the atmosphere exerts a force of 14.7 pounds per square inch (psi) on any object placed on the surface. The following is a breakdown of the conversion factors for each unit of pressure measurement:1 atm = 760 torr1 atm = 760 mmHg1 atm = 101.3 kPa1 atm = 101,300 Pa1 atm = 14.7 psiTorr and mmHg are commonly used in vacuum technology, and they refer to the pressure exerted by a mercury column in a manometer at 0 °C. KPa and Pa are metric units of pressure that are widely used in scientific research, while psi is a unit of pressure primarily used in the United States and the United Kingdom to measure tire pressure and other similar applications.
for such more questions on pressures
https://brainly.com/question/24719118
#SPJ8
Which of the following physical changes is not an example of a change in state?
A. rock breaking with a hammer C. corn with ice
B. a (ball looking) object into water D. water filling into a ice cube mold
Answers
Answer:
Option
Explanation:
Rock is broken into piece but thestate is normal
How can you show the conservation of mass when making a physical mixture?
A.
Show that the total of the masses of the starting substances equals the mass of the mixture.
B.
Show that the total of the masses of the starting substances is greater than the mass of the mixture.
C.
Show that the mass of some of the substances changes during mixing.
D.
Show that the mass of the mixture depends on how quickly the substances are combined.
THERE IS MORE THAN ONE ANSWER
Answers
Answer:
A. Show that the total of the masses of the starting substances equals the mass of the mixture.
Explanation:
Law of conservation of mass:
According to the law of conservation mass, mass can neither be created nor destroyed in a chemical equation.
This law was given by French chemist Antoine Lavoisier in 1789. According to this law mass of reactant and mass of product must be equal, because masses are not created or destroyed in a chemical reaction.
For example:
In given photosynthesis reaction:
6CO₂ + 6H₂O + energy → C₆H₁₂O₆ + 6O₂
there are six carbon atoms, eighteen oxygen atoms and twelve hydrogen atoms on the both side of equation so this reaction followed the law of conservation of mass.
In a similar way if we have mixture of sugar and sand the total weight of mixture is equal to the weight of reactants.
sand + sugar + water → mixture
10g+5g+20 g = 35 g
Which of these is a sign of salinization in crop plants?
A. Yellowing of leaves
B. Wilting of seedlings
C. Dead patches on leaves
D. Enlarged seedlings
Answers
Answer:
The correct answer I think is Wilting of Seeds
Explanation:
salinization is when the water evaporates and leaves behind the salt in the water. Which causes the seed to whither because salt sits in its roots, and leaves no way for any more water to get in. So it makes it hard for seedling so get water.
I think that is correct! Hope its right
A sign of salinization in crop plants is Wilting of seedlings. Thus the correct option is B.
What is salinization ?Increased salt percentage in the soil is known as salinization, and it is brought on by soluble substances in the water supply. The land may be flooded by seawater or saline groundwater flowing through the soil downward, depending on the source of the water.
Due to the poor plant growth circumstances caused by salinity, the ground surface is constantly moist and lacks vegetation. Lands become very prone to erosion as a consequence. The amount of salt on the soil's surface rises as a result of excessive irrigation owing to evaporation.
Saline soils cause crops to suffer because of high salinity stress, nutritional problems and toxins, poor soil physical conditions, and decreased agricultural output.
Therefore, option B is appropriate.
Learn more about salinization, here:
https://brainly.com/question/5082030
#SPJ2