Answers
Answer:
Paraffin oil is used for determination of boiling point and melting point for the following reasons: It has a very high boiling point and so it can be used to maintain high temperatures in the boiling and melting point apparatus without loss of the substance.
Related Questions
Calculate the pH for the following concentration: [H3O+] = 5.77 x 10-7 M
Answers
The pH of a solution with a concentration of 5.77 x 10-⁷ M is 6.24.
How to calculate pH?pH refers to the quantitative measure of the acidity or basicity of aqueous or other liquid solutions.
The pH of a substance can be calculated using the following expression:
pH = -log H
Where;
H = concentration of acidAccording to this question, the concentration of a solution is 5.77 × 10-⁷M. The pH can be calculated as follows:
pH = - log 5.77 × 10-⁷M
pH = 6.24
Therefore, 6.24 is the pH of the solution
Learn more about pH at: https://brainly.com/question/15289741
#SPJ1
The gravity battery is similar to an but it doesn’t carry people
Answers
Answer:i try and try but nobody likes me
Explanation: that's the story of the day lucky person brainiest pls
hehehhehehhehehehehehhehhehehehehehehehehehehe
4. The volume of a liquid sample is measured as 15.43 L. We need to know the volume in
mL.
3.
b.
What conversion factor would be used in the calculation?
Calculate the volume in mL.
Answers
Conversion factor used to convert volume is 1L = 1000 mL.
15.43L = 15430mL
The same attribute is expressed using a unit conversion but in a different unit of measurement. For instance, time can be expressed in minutes rather than hours, and distance can be expressed in kilometers, feet, or any other measurement unit instead of miles. Measurements are frequently offered in one set of units, like feet, but are required in another set, like chains. A conversion factor is a mathematical equation that facilitates an equal exchange of feet for chains.
A conversion factor is a number that is used to multiply or divide one set of units into another. If a conversion is required, it must be done using the correct conversion factor to get an identical value.
To learn more about the Conversion factors please visit-
https://brainly.com/question/28366871
#SPJ9
Compare and contrast electron behavior in Rutherford’s, Bohr’s, and the quantum models
Answers
Rutherford’s, Bohr’s, and the quantum models say that electrons behave as particles, electron is negatively charged and electrons behave both particles and wave respectively.
What Rutherford’s, Bohr’s, and the quantum models say about electron?Bohr model states that electrons behave as particles, Rutherford's model proposed that the negatively charged electrons surround the nucleus of an atom whereas quantum model explains that the electron has both particle and wave behavior.
So we can conclude that Rutherford’s, Bohr’s, and the quantum models say that electrons behave as particles, electron is negatively charged and electrons behave both particles and wave respectively.
Learn more about electron here: https://brainly.com/question/860094
#SPJ1
what is the mole fraction of sulfuric acid in a solution made by adding 3.4 g of sulfuric acid to 3500 ml of water ?
Answers
The mole fraction of sulfuric acid in the solution is 1.78 x 10^-4.
We need to know how many moles of sulfuric acid and how many moles there are in the solution as a whole in order to figure out the mole fraction of sulfuric acid.
To begin, we must convert the sulfuric acid's mass into moles. The molar mass of sulfuric corrosive is 98.08 g/mol. As a result, the number of moles of sulfuric acid is equal to 3.4 g divided by 98.08 g/mol, or 0.0347 mol.
Next, we need to figure out how many moles are all around the solution. We can expect that the volume of the arrangement is equivalent to the volume of water added (3500 ml). Notwithstanding, we want to switch the volume from milliliters over completely to liters since the unit of mole portion is moles per liter.
As a result, the volume of the solution is 3500 ml, or 3.5 L. Based on the assumption that water has a density of 1 g/mL, the mass of water in the solution can be calculated as follows:
The molar mass of water, which is 18.02 g/mol, can be used to determine the number of moles of water: mass of water = volume of water x density of water = 3500 ml x 1 g/mL = 3500 g
The mole fraction of sulfuric acid in the solution can be calculated as follows: 3500 g x 18.02 g/mol = 194.14 mol
Mole fraction of sulfuric acid is calculated by dividing the total number of moles in the solution by the number of moles of sulfuric acid: 0.0347 mol / (0.0347 mol + 194.14 mol) = 1.78 x 10-4.
Therefore, the mole fraction of sulfuric acid in the solution is 1.78 x 10^-4.
For more such questions on mole fraction
https://brainly.com/question/28019441
#SPJ11
30 points please help
which kind of energy is stored in the bonds between molecules that make up food?
kinetic energy
potential energy
thermal energy
chemical energy
Answers
Answer:
Chemical Energy is stored in food molecules
Answer:
chemical energy
Explanation:
because none of the other make sense
What is the electron geometry and hybrid orbital of sio2
Answers
Answer:
linear electron and molecular geometry with a bond angle of 180 degrees and a hybridization of Sp
Explanation:
Estimate the volume of a solution of 5M NaOH that must be added to adjust the pH from 4 to 9 in 100 mL of a 100 mM solution of a phosphoric acid?
Answers
Answer:
3mL of 5M NaOH must be added to adjust the pH to 7.20
Explanation:
When NaOH is added to phosphoric acid, H₃PO₄, the reaction that occurs are:
NaOH + H₃PO₄ ⇄ NaH₂PO₄ + H₂O pKa1 = 2.15
NaOH + NaH₂PO₄ ⇄ Na₂HPO₄ + H₂O pKa2 = 7.20
NaOH + Na₂HPO₄ ⇄ Na₃PO₄ + H₂O pKa3 = 12.38
We can adjust the pH at 7.20 = pKa2 if NaH₂PO₄ = Na₂HPO₄. To make that, we must convert, as first, all H₃PO₄ to NaH₂PO₄ and the half of NaH₂PO₄ to Na₂HPO₄. To solve this question we need to find the moles of phophoric acid in the initial solution. 1.5 times these moles are the moles of NaOH that must be added to fix the pH to 7.20:
Moles H₃PO₄:
100mL = 0.100L * (0.100mol / L) = 0.0100 moles H₃PO₄
Moles NaOH:
0.0100 moles H₃PO₄ * 1.5 = 0.0150 moles NaOH
Volume NaOH:
0.0150 moles NaOH * (1L / 5moles) = 3x10⁻³L 5M NaOH are required =
3mL of 5M NaOH must be added to adjust the pH to 7.203 mL of 5 Molar NaOH is required to adjust the pH of phosphoric acid.
What is pH?It is the negative log of the concentration of Hydrogen ions in the solution.
To calculate the volume of NaOH first, calculate the moles of NaOH and H₃PO₄.
Moles of H₃PO₄.
\(\rm moles \ of \ H_3PO_4 = 100\rm \ mL = 0.100\rm \ L \times (0.100 \ mol / L)\\\\\rm moles \ of \ H_3PO_4 = 0.01\)
The moles of NaOH:
\(\rm Moles \ of \ NaOH =0.01 \ moles \ H_3PO_4\times 1.5 \\\\\rm Moles \ of \ NaOH= 0.0150\)
The volume of NaOH:
\(\rm Volume\ of \ NaOH = \rm 0.0150\ moles\ NaOH \times (1 \ L / 5 \ moles) \\\\\rm Volume\ of \ NaOH = 3\times 10^{-3} L\)
Therefore, 3 mL of 5 Molar NaOH is required to adjust the pH of phosphoric acid.
Learn more about pH:
https://brainly.com/question/3262317
0°C in 101.3 K PA pressure a sample of gas occupies 30 mL if the temperature is increased to 30°C in the entire gas sample is transferred to a 20 mL container what will be the gas pressure inside a container
Answers
Answe 174.0 KPA
Explanation:
0°C in 101.3 K PA pressure a sample of gas occupies 30 mL if the temperature is increased to 30°C in the entire gas sample is transferred to a 20 mL container what will be the gas pressure inside a container
PV=nRTt
so
n = PV/RT
P = 101.3 KPA
T = 0+273.2 = 273.200
V = (30/1000) L = 0.03 L
R =0.082
SO
n= (101.3 X 0.03)/(0.082 X 273.2)
= 0.14 moles
PV= nRT
N= 0,14
T = 30 + 273.2= 303.2
V= (20/1000)L =0.02L
since PV = nRT
THEN
P=nRT/V
so
P=(0.14 X 0.082 X 303.2)/0.02 =
174.0 KPA
What is represented by a straight line on a graph?
o the sum of the independent and dependent variables
O only the independent variable
O only the dependent variable
o the relationship between independent and dependent variable
1 2
3
4
5
Answers
Answer:
the relationship between independent and dependent variable
Explanation:
A straight line or linear graph is one of the ways to represent a given data. It shows the relationship between two given set of data; one called the independent variable is plotted on the x-axis (horizontal) while the other called the dependent variable is plotted on the y-axis (vertical).
The straighter the line is, the stronger the relationship between the two variables and vice versa. Hence, the straight line in the graph represents the relationship between independent and dependent variable.
The diagram shows different forms of energy transfered. What do the flames below the pot represent
Answers
Answer:
That it is cooking the food or whatever is in the pot.
Learning this in science.
g h g jg jh gj h g jhg u iga ff f. f. f f f
Answers
What happens to the electrons when an electric field is applied?
Answers
When electric voltage is applied, an electric field within the metal triggers the movement of the electrons, making them shift from one end to another end of the conductor
Explanation:
When electric voltage is applied, an electric field within the metal triggers the movement of the electrons, making them shift from one end to another end of the conductor
A large office building is 1.07 102 m long, 31 m wide, and 4.25 x 102 m high. What is its volume?
Answers
Answer:
1409725 m^3
Explanation:
volume = length * width * height
volume = (1.07e2) * (31) * (4.25e2)
volume = 1409725 m^3
The mass of an atom of element x is equivalent to the total mass of 7 hydrogen atoms

Answers
Answer: I'm not sure what your question is but i'll answer as best as I can.
Explanation:
Since X is equal to 7 H and we know that H is equal to 1.008, we can just do 7x1.008 = 7.056 g. I hope i helped and please clarify more in the future.
The atomic mass of an element is given by the sum of the mass of protons and neutrons. The element X is lithium as it has a mass of 6.941 u.
What is atomic mass?Atomic mass is the sum of the masses of the isotopes of that element and is given by adding the masses of the number of protons and neutrons of the elemental atom.
The atomic mass of one hydrogen atom is 1.008, so seven hydrogens will be, 7 x 1.008 = 7.056 g. Now, from the periodic table, it can be seen that an atomic mass of 7.056 g is closest to the atomic mass of a lithium atom (6.941 u).
From the mass, it can be said that seven hydrogen atom has an equivalent mass as that of one lithium atom in the periodic table. The lithium atom has atomic number 3 and an atomic mass of 6.941 g/mol.
Therefore, element X is lithium.
Learn more about atomic mass here:
https://brainly.com/question/17067547
#SPJ5
The Kp for the reaction A (g) ⇌ 2 B (g) is 0.0110. What is Kp for the reaction 2 B (g) ⇌ A (g)?
Answers
The United States experienced a decrease in the real GDP, high inflation, and a
rising unemployment rate. The United States
was in the middle of an economic boom
appeared to be entering a recession
was in an economic slump
was in a stagnant economic period
Answers
The United States experienced a decrease in the real GDP, high inflation, and arising unemployment rate.
The United States appeared to be entering a recession.A recession is a decline in economic activity, characterized by declining GDP, high unemployment rates, and increased unemployment benefits. Economic analysts and the media commonly use a two-quarter consecutive decline in real GDP as a definition of a recession.
The United States is considered to have entered a recession in the 1970s, which was characterized by an energy crisis, inflation, and recession. However, by the end of the decade, the economy had improved, and it entered into the 1980s with a strong economic performance.
The 1970s were a period of high inflation, low growth, and an oil crisis, which had a significant impact on the United States economy. Therefore, it can be concluded that The United States was in the middle of an economic boom before the 1970s recession and entered a recession in the 1970s due to a decrease in the real GDP, high inflation, and arising unemployment rate.
For more such questions on inflation visit;
https://brainly.com/question/28061405
#SPJ8
Is natural gas the most harmful fossil fuel to the environment?
Answers
There are different approachs to this question, but in my opinion I think that natural gas is not the most harmful fossil fuel to the environment.
Since natural gas is Methane (CH₄) it burns cleaner than other fussil fuels like coal for example.
Natural Gas 0.2 kg CO₂/kWh
Gasoline 0.25 kg CO₂/kWh
Fuel Oil 0.28 kg CO₂/kWh
There we have 3 fossil fuels. If we look at them we will see that if we burn Natural Gas we can produce more energy with less emissions of Carbon Dioxide.
Convert 6.75 g Al to moles.
Answers
Answer:
\(\huge\boxed{\sf n = 0.25\ moles}\)
Explanation:
Given data:Mass in g = m = 6.75 g
Molar mass of Al = M = 27 g/mol
Required:No. of moles = n = ?
Formula:n = m / M
Solution:Put the given data in the above formula.
n = 6.75 / 27
n = 0.25 moles\(\rule[225]{225}{2}\)
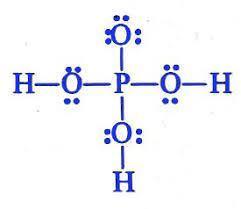
Which is the Lewis structure for H3PO4? An upper P is single bonded above to an O, and to the left, right, and below to an O single bonded to an H. The O above the P has three pairs of dots to the left, above, and below; the O's to the sides have pairs of dots above and below, and the O below the P has pairs of dots right and left. A central upper P is single bonded left, right, above, and below to upper Os. The O above the P is single bonded to upper H on the left and the right, and has two electron dots above it. The O below the P is single bonded to an H below, and has pairs of electron dots to the left and right. A central upper P is double bonded to an O above, and single-bonded to an upper O single-bonded to an upper H to the left and the right. The O above the P has three pairs of electron dots, to the left, above, and to the right; the O's to the right and left have pairs of dots above and below. A central upper P is bonded to an upper H above, an upper O below, and upper O's bonded to upper H's to the left and the right. The O below the P has three pairs of electron dots, to the left, right, and below; the other two O's have pairs of dots above and below. A central upper P is double bonded to an O above, and single-bonded to an upper O single-bonded to an upper H to the left and the right. The O above the P has three pairs of electron dots, to the left, above, and to the right; the O's to the right and left have pairs of dots above and below.
Answers
Answer:
It is A.
Explanation:
I took the test.
The Lewis structure shows the arrangement of valence electrons in H3PO4.
The Lewis structure gives us a picture of the number of valence electrons in a molecule. This is because, in a Lewis structure, the electrons in the molecule are shown as dots. A single line may be used to show shared electrons in a covalent bond.
The correct Lewis structure of H3PO4 is an upper P is single bonded above to an O, and to the left, right, and below to an O single bonded to an H. The O above the P has three pairs of dots to the left, above, and below; the O's to the sides have pairs of dots above and below, and the O below the P has pairs of dots right and left.
Learn more: https://brainly.com/question/4144781
What is the pH of a 0.0046 M nitric acid solution
Answers
Answer: 2.34
Explanation:
pH= -log (H+)
H+ is the acid's M
pH= -log (0.0046) =2.34
How many calcium atoms will you need to balance this: Ca(PO4)
Answers
Answer: 2
Explanation: Calcium has a +2 charge and PO4 has a -1 charge. When writing this expression, the charges are flipped; instead of Ca2(PO4), it's Ca(PO4)2.
Did all the silver ions get consumed in the reaction? The molar mass of silver is 107.87 g/mol. Justify your answer.
Answers
This problem is asking for the consumption of silver ions when silver nitrate is reacted with copper. In such a case, since no masses are given, we can use the following from similar problems:
Mass of empty beaker: 110.000 g
Mass of beaker with silver nitrate (after all additions) and copper: 331.634 g.
Mass of beaker with silver: 113.395 g.
This means we can write the following chemical equation:
\(Cu+2AgNO_3\rightarrow Cu(NO_3)_2+2Ag\)
And thus calculate the mass of silver nitrate that will produce the following mass of silver:
\(m_{Ag}^{produced}=113.395g-110.000g=3.395g\)
Next, we use the 2:2 mole ratio of silver to silver nitrate (silver ions source):
\(3.395gAg*\frac{1molAg}{107.87gAg}*\frac{2molAgNO_3}{2molAg} *\frac{169.87gAgNO_3}{1molAgNO_3} = 5.35gAgNO_3\)
The step will be defined for the given mass of available silver nitrate which will be compared to 5.35 g (consumed mass) to see if they are the same (all consumed) or different (partial consumption).
Learn more:
https://brainly.com/question/22031122https://brainly.com/question/2607181https://brainly.com/question/16965188Polar water molecules can surround ions, reducing the likelihood of them interacting with other ions. What property of water does this phenomenon cause?
Answers
The property of water that causes polar water molecules to surround ions, reducing the likelihood of them interacting with other ions, is known as its solvation or hydration ability.
This solvation property is a result of water's high polarity, which arises from its asymmetrical molecular structure and the presence of polar covalent bonds. Water molecules have a partially positive (+) and partially negative (-) end due to the electronegativity difference between oxygen and hydrogen atoms. This polarity enables water molecules to form hydrogen bonds with other water molecules and with polar solutes, such as ions. When ions dissolve in water, the partially positive hydrogen atoms of water molecules are attracted to the negatively charged ions, and the partially negative oxygen atoms of water molecules are attracted to the positively charged ions.
Learn more about the hydrating nature of water here.
https://brainly.com/question/2709403
#SPJ1
4. Consider Limitations Students were given the following sets of items and asked, for each set, to identify which item had greater thermal energy. For which set would this task be most difficult? Explain. Set 1: 5 g of iron at 45°C and 10 g of iron at 45 °C Set 2: 10 g of water at 30°C and 10 g of water at 50°℃ Set 3: 32 g of copper at 50°C and 32 g of nickel at 50°C
Answers
Set 1 is difficult.
The formula for thermal energy is given as:
Q = mcΔT
Where m is the mass of the item, c is specific heat capacity and ΔT is the change in temperature when the item is heated.
For Set 1:
5 g of iron at 45°C and 10 g of iron at 45 °C
In this case, the mass of iron is different at the same temperature. Since there is no change in temperature hence the metal is not heated. Another reason is that the mass of iron is different in this case. Hence, this set will be difficult to find thermal energy.
Set 2:
10 g of water at 30°C and 10 g of water at 50° C
The mass of water at both temperatures is the same, and the water is heated from 30°C to 50° C The thermal energy can be easily calculated using the formula Q = mcΔT.
Set 3:
2 g of copper at 50°C and 32 g of nickel at 50°C
The mass of copper remains unchanged, also the temperature doesn't change. Since copper is not heated and the temperature remains constant. Hence the thermal energy will be zero.
Therefore, Set 1 will be the most difficult to find the thermal energy.
Learn more about thermal energy here:
https://brainly.com/question/19666326
#SPJ9
explain how the atom developed over time through the experiments done by Thomason, Ruthford, Bohr, and Schrodinger
Answers
ANSWER
EXPLANATION
An atom is defined as the smallest indivisible particle of an element that can take part in a chemical reaction and still retain its properties.
There are three sub-atomic particles which are; electron, proton, and neutron.
J.J Thomson contributed to the development of the atomic theory when it performed an experiment on cathode ray tubes.
The experiment shows that all atoms contain tiny negatively charged called electron. This led to the discovery of electron
Rutherford contributed to the development of the atomic theory when he discovered that the atom is mostly empty space, nearly all the mass of the atom is concentrated in a tiny central nucleus. He also found out that the nucleus is positively charged and this led to the discovery of proton
Niels Bohr contributed to the development of the atomic theory when he proposed a model of the atom in which the electron was able to occupy only certain orbit around the nucleus. This model was the first to use quantum theory.
Schrondinger contributed to the development of the atomic theory when he assumed that matter can behave as both particles and waves.
Assess It! Question #2: Which is not true about the Law of Conservation of Mass? A: Nothing is created B: The same number of atoms are in both the products and reactants C: New atoms are created when a new substance is formed D: Nothing is destroyed
Answers
Option C) New atoms are created when a new substance is formed is not true about the Law of Conservation of Mass.
The Law of Conservation of Mass states that "Mass can neither be created nor be destroyed". This means that the mass of an object will remain the same, regardless of the changes that occur to its form or composition. The law is a fundamental principle of physics and chemistry and is used to calculate the amount of matter that is present in a given sample.
The same number of atoms are in both the products and reactants as the mass of the reaction is conserved.
Since the same number of atoms are present in both reactants and products, then no new atoms or compounds are created in the process.
Hence, the correct option is C).
To know more about Law of conservation of momentum, click below:
https://brainly.com/question/7538238
#SPJ9
The amount of space that matter takes up is its
Answers
Answer:
Mass is the amount of mass an object contains. Mass is measured using a scale. Volume is the amount of space matter takes up. Hope this helps you get an idea in your head!
Explanation:
A 5.00g of X, the product of organic synthesis is obtained in a 1.0 dm3 aqueous solution. Calculate the mass of X that can be extracted from the aqueous solution by a 50cm3 of ethoxy ethane. (KD (X) =40.
Answers
Answer:
mass of X extracted from the aqueous solution by 50 cm³ of ethoxy ethane = 3.33 g
Explanation:
The partition coefficient of X between ethoxy ethane (ether) and water, K is given by the formula
K = concentration of X in ether/concentration of X in water
Partition coefficient, K(X) between ethoxy ethane and water = 40
Concentration of X in ether = mass(g)/volume(dm³)
Mass of X in ether = m g
Volume of ether = 50/1000 dm³ = 0.05 dm³
Concentration of X in ether = (m/0.05) g/dm³
Concentration of X in water = mass(g)/volume(dm³)
Mass of X in water left after extraction with ether = (5 - m) g
Volume of water = 1 dm³
Concentration of X in water = (5 - m/1) g/dm³
Using K = concentration of X in ether/concentration of X in water;
40 = (m/0.05)/(5 - m)
(m/0.05) = 40 × (5 - m)
(m/0.05) = 200 - 40m
m = 0.05 × (200 - 40m)
m = 10 - 2m
3m = 10
m = 10/3
m = 3.33 g of X
Therefore, mass of X extracted from the aqueous solution by 50 cm³ of ethoxy ethane = 3.33 g
Suppose Gabor, a scuba diver, is at a depth of 15m15m. Assume that: The air pressure in his air tract is the same as the net water pressure at this depth. This prevents water from coming in through his nose. The temperature of the air is constant (body temperature). The air acts as an ideal gas. Salt water has an average density of around 1.03 g/cm3g/cm3, which translates to an increase in pressure of 1.00 atmatm for every 10.0 mm of depth below the surface. Therefore, for example, at 10.0 mm, the net pressure is 2.00 atmatm. What is the ratio of the molar concentration of gases in Gabor's lungs at the depth of 15 meters to that at the surface
Answers
Answer:
The ration of molar concentration is "2.5".
Explanation:
The given values are:
Average density of salt water,
= \(1.03 \ g/cm^3\)
Net pressure,
= \(2.00 \ atm\)
Increase in pressure,
= \(1.00 \ atm\)
Now,
The under water pressure will be:
= \(\frac{15 \ m}{10}\times 1 \ atm +1 \ atm\)
= \(1.5\times 1+1\)
= \(1.5+1\)
= \(2.5 \ atm\)
hence,
The ratio will be:
= \(\frac{(\frac{n}{V})_{15m} }{(\frac{n}{V})_{surface} }\)
or,
= \(\frac{P}{P_s}\)
= \(\frac{2.5}{1}\)
= \(2.5\)